Question: Match the thermodynamic processes given under Column I with the expression given under Column II: Column I Column II (A) Freezing of water at
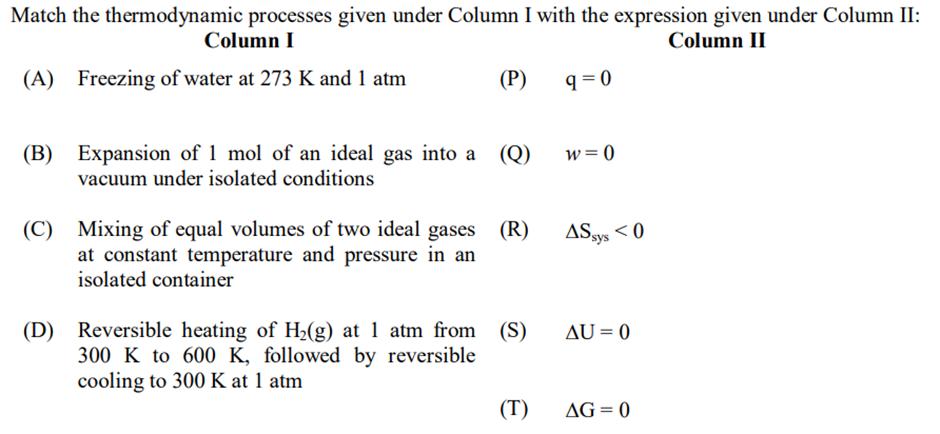
Match the thermodynamic processes given under Column I with the expression given under Column II: Column I Column II (A) Freezing of water at 273 K and 1 atm (P) (B) Expansion of 1 mol of an ideal gas into a (Q) vacuum under isolated conditions (C) Mixing of equal volumes of two ideal gases at constant temperature and pressure in an isolated container (R) (D) Reversible heating of H(g) at 1 atm from (S) 300 K to 600 K, followed by reversible cooling to 300 K at 1 atm (T) 9=0 w=0 AS sys
Step by Step Solution
3.38 Rating (157 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


