Question: Material and energy balance problem Appendix - A mixture of 60% ethane and 40% propane has a flowrate of 150 mol/h. Calculate the heat required

Material and energy balance problem
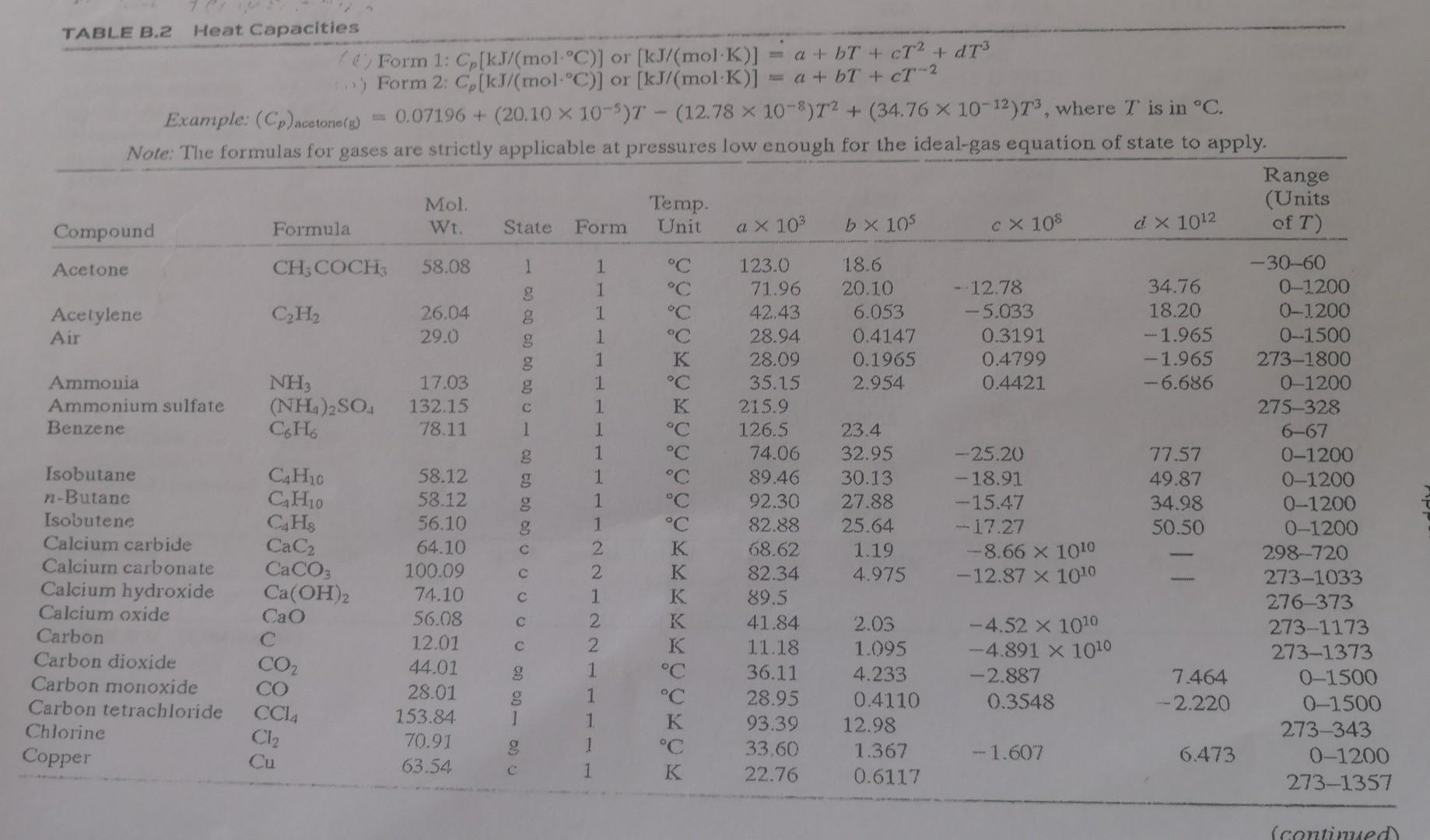
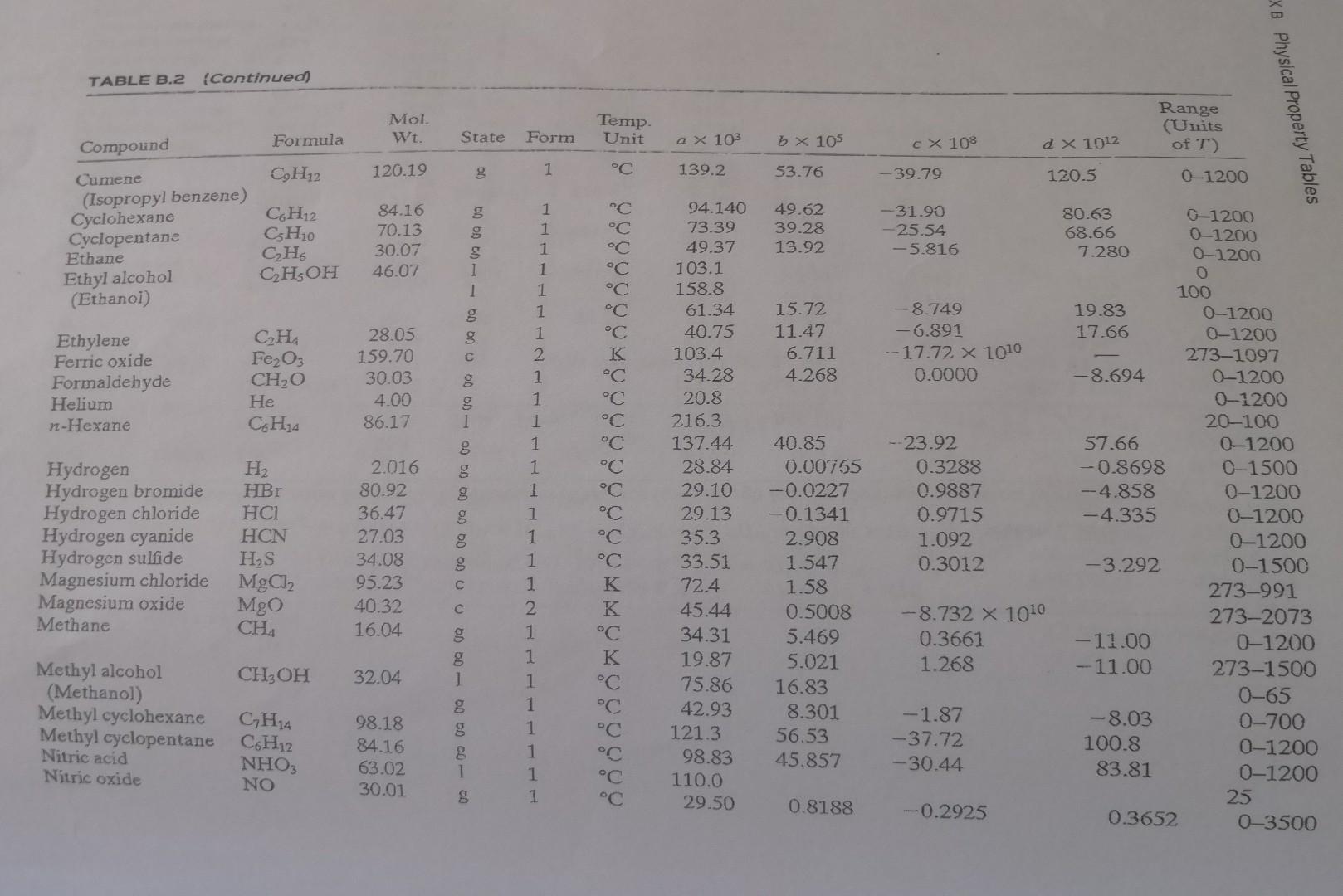
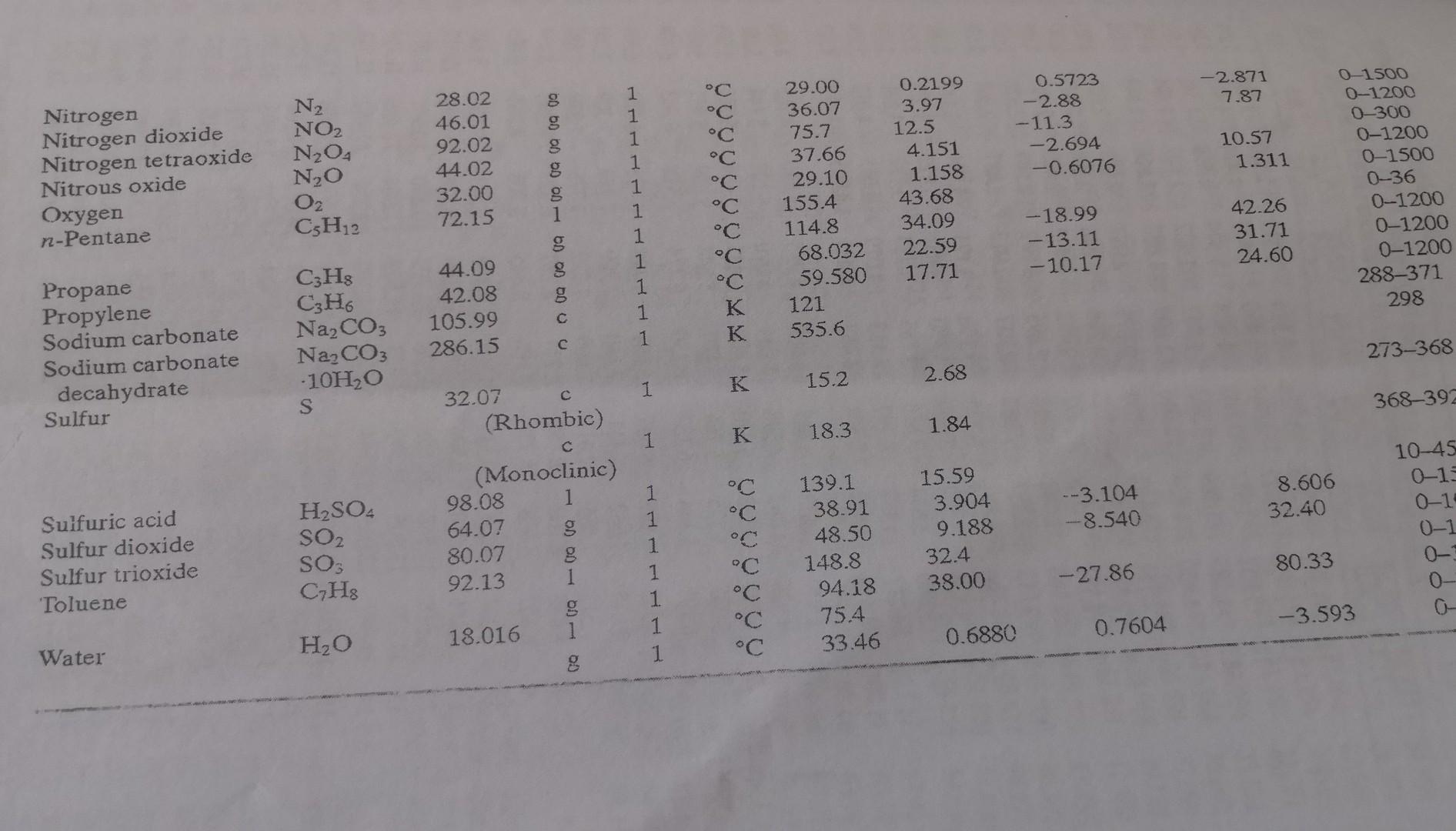
Appendix
- A mixture of 60% ethane and 40% propane has a flowrate of 150 mol/h. Calculate the heat required (0) needed to increase the mixture temperature from 0C to 300C. Your answer should be in kJ/h. a x 10 C X 10$ C TABLE B.2 Heat Capacities Form 1: Cp[kJ/mol.C)] or [kJ/mol K)] = a + b + cT2 + dt3 Form 2: C (kJ/mol.C)] or [kJ/(mol K)] a + b + cT-2 Example: (Cp)ocatore) = 0.07196 + (20.10 x 10-5) - (12.78 x 10-)T2 + (34.76 x 10-12)73, where T is in C. Note: The formulas for gases are strictly applicable at pressures low enough for the ideal-gas equation of state to apply. Range Mol Temp. (Units Compound Formula Wt. State Form Unit b X 105 d! X 1012 of T) Acetone CH3COCH 58.08 1 1 C 123.0 18.6 -30-60 1 C 71.96 20.10 -12.78 34.76 0-1200 Acetylene CH 26.04 8 1 42.43 6.053 -5.033 18.20 0-1200 Air 29.0 g 1 C 28.94 0.4147 0.3191 - 1.965 0-1500 1 28.09 0.1965 0.4799 -1.965 273-1800 Ammonia NH 17.03 g 1 35.15 2.954 0.4421 -6.686 0-1200 Ammonium sulfate (NH4)2SO4 132.15 1 K 215.9 275-328 Benzene 66 78.11 1 1 C 126.5 23.4 6-67 8 1 C 74.06 32.95 -25.20 77.57 0-1200 Isobutane CH20 58.12 g 1 C 89.46 30.13 -18.91 49.87 0-1200 n-Butane Ca Ho 58.12 1 "C 92.30 27.88 - 15.47 34.98 0-1200 Isobutene CqHs 56.10 1 C 82.88 25.64 - 17.27 50.50 0-1200 Calcium carbide CaC2 64.10 2 K 68.62 1.19 --8.66 X 1010 298-720 Calcium carbonate CaCO3 100.09 2 K 82.34 4.975 -12.87 X 1010 273-1033 Calcium hydroxide Ca(OH)2 74.10 1 K 89.5 276-373 Calcium oxide Cao 56.08 2 K 41.84 2.03 -4.52 x 1010 Carbon 273-1173 12.01 2 K 11.18 1.095 -4.891 X 1010 Carbon dioxide 273-1373 CO2 44.01 1 C 36.11 4.233 Carbon monoxide -2.887 7.464 0-1500 CO 28.01 1 C 28.95 Carbon tetrachloride 0.4110 0.3548 CCL --2.220 0-1500 153.84 1 K Chlorine 93.39 12.98 273-343 70.91 1 C 33.60 Copper 1.367 Cu -1.607 6.473 0-1200 63.54 1 K 22.76 0.6117 273-1357 00 00 1 40 - 00 Cl C (comed TABLE B.2 (Continued) XB Physical Property Tables Mol. Wt. Range (Units of T) State Temp. Unit Form a X 103 b x 105 CX 108 2 x 1012 120.19 g 1 C 139.2 53.76 - 39.79 120.5 01200 g Compound Formula Cumene CH12 (Isopropyl benzene) Cyclohexane CH12 Cyclopentane CH0 Ethane CH, Ethyl alcohol CH5OH (Ethanoi) 84.16 70.13 30.07 46.07 49.62 39.28 13.92 -31.90 -25.54 -5.816 s 1 1 Ethylene Ferric oxide Formaldehyde Helium n-Hexane CHa Fe2O3 CH2O He C. HjA C C C C C C C K C C C C C 28.05 159.70 30.03 4.00 86.17 15.72 11.47 6.711 4.268 -8.749 -6.891 -17.72 X 1010 0.0000 g 1 g Hydrogen Hydrogen bromide Hydrogen chloride Hydrogen cyanide Hydrogen sulfide Magnesium chloride Magnesium oxide Methane H2 HBr HCI HCN HS MgCl2 Mgo CHA 2.016 80.92 36.47 27.03 34.08 95.23 40.32 16.04 g g 8 1 1 1 1 1 1 1 2 1 1 1 1 1 1 1 1 1 1 2 1 1 1 1 1 1 1 1 94.140 73.39 49.37 103.1 158.8 61.34 40.75 103.4 34.28 20.8 216.3 137.44 28.84 29.10 29.13 35.3 33.51 72.4 45.44 34.31 19.87 75.86 42.93 121.3 98.83 110.0 29.50 --23.92 0.3288 0.9887 0.9715 1.092 0.3012 80.63 0-1200 68.66 0-1200 7.280 0-1200 0 100 19.83 0-1200 17.66 0-1200 273-1097 -8.694 0-1200 0-1200 20-100 57.66 0-1200 -0.8698 0-1500 -4.858 0-1200 -4.335 0-1200 0-1200 -3.292 0-1500 273-991 273-2073 - 11.00 0-1200 - 11.00 273-1500 0-65 -8.03 0-700 100.8 0-1200 83.81 0-1200 25 0.3652 0-3500 40.85 0.00755 -0.0227 -0.1341 2.908 1.547 1.58 0.5008 5.469 5.021 16.83 8.301 56.53 45.857 C K K C K C C C -8.732 X 1010 0.3661 1.268 8 CH3OH 32.04 DOO OD 0 - 00 00 00 00 Methyl alcohol (Methanol) Methyl cyclohexane Methyl cyclopentane Nitric acid Nitric oxide g GH4 C6H12 NHO NO 98.18 84.16 63.02 30.01 -1.87 -37.72 -30.44 g . 0.8188 -0.2925 C -2.871 7.87 g N2 NO2 NO4 NO 02 CH2 Nitrogen Nitrogen dioxide Nitrogen tetraoxide Nitrous oxide Oxygen n-Pentane 28.02 46.01 92.02 44.02 32.00 72.15 0.5723 -2.88 -11.3 -2.694 -0.6076 10.57 1.311 00 60 OD OD 0 DO 1 1 1 1 1 1 1 1 1 1 1 C C C C C C C K K 29.00 36.07 75.7 37.66 29.10 155.4 114.8 68.032 59.580 121 535.6 0.2199 3.97 12.5 4.151 1.158 43.68 34.09 22.59 17.71 0-1500 0-1200 0-300 0-1200 0-1500 0-36 0-1200 0-1200 0-1200 288-371 298 -18.99 -13.11 -10.17 42.26 31.71 24.60 C3H8 CH6 Na2CO3 Na2CO3 - 10H2O S 44.09 42.08 105.99 286.15 Propane Propylene Sodium carbonate Sodium carbonate decahydrate Sulfur 273-368 15.2 2.68 K 1 368-392 32.07 (Rhombic) 18.3 1.84 1 K 8.606 32.40 --3.104 -8.540 10-45 0-15 0-1 0-1 Sulfuric acid Sulfur dioxide Sulfur trioxide 'Toluene H2SO4 SO2 SO CH: (Monoclinic) 98.08 1 64.07 80.07 g 92.13 g 18.016 1 1 1 1 1 1 15.59 3.904 9.188 32.4 38.00 C C C C C C C 139.1 38.91 48.50 148.8 94.18 75.4 33.46 80.33 -27.86 -3.593 H2O 0Q (Q 0.7604 0.6880 Water 1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


