Question: material science Question 1 The lattice constant for BCC Vanadium at 20C is 0.3039nm and its density is 5.96g/cm3. Calculate a value for its atomic

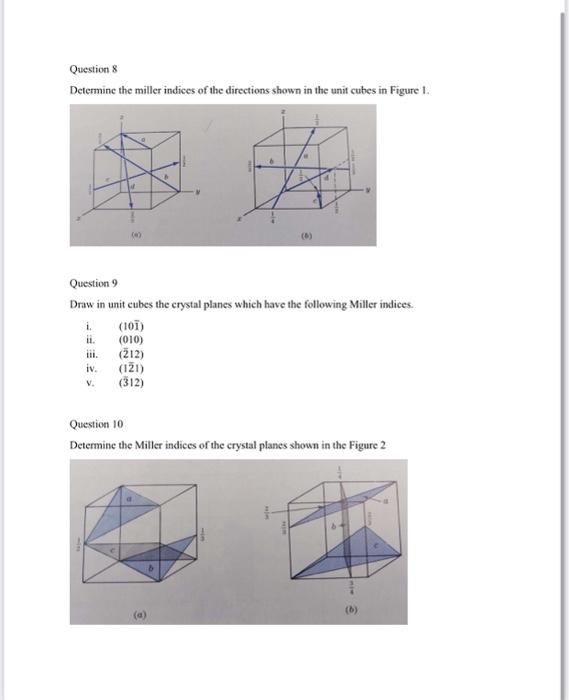
Question 1 The lattice constant for BCC Vanadium at 20C is 0.3039nm and its density is 5.96g/cm3. Calculate a value for its atomic mass. [Ans: 50.4g/mol] Question 2 Calculate the planar atomic density in atoms per square millimeter for the following crystal planes in FCC copper, which has a lattice constant of 0.3615nm. (a) (100); (b) (110); (c) (111) Question 3 Calculate the linear atomic density in atoms per millimeter for the following directions in BCC tungsten, which has a lattice constant of 0.3158nm. (a) [100]; (b) [110]; (c) [111] Question 4 Show that the atomic packing factor for HCP is 0.74 Question 5 Calculate the radius of an iridium (Ir) atom, given that Ir has an FCC crystal structure, a density of 22.4g/cm3, and an atomic weight of 192.2g/mol. Question 6 If the atomic radius of aluminum is 0.143nm, calculate the volume of its unit cell in cubic meters Question 7 Draw direction vectors in unit cells for the following cubic directions i. [111] ii. [110] iii. [121] iv. [ 113] Question 8 Determine the miller indices of the directions shown in the unit cubes in Figure 1. Question 9 Draw in unit cubes the erystal planes which have the following Miller indices. i. (101) ii. (010) iii. (2) (2) iv. (121) v. (312) Question 10 Determine the Miller indices of the crystal planes shown in the Figure 2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


