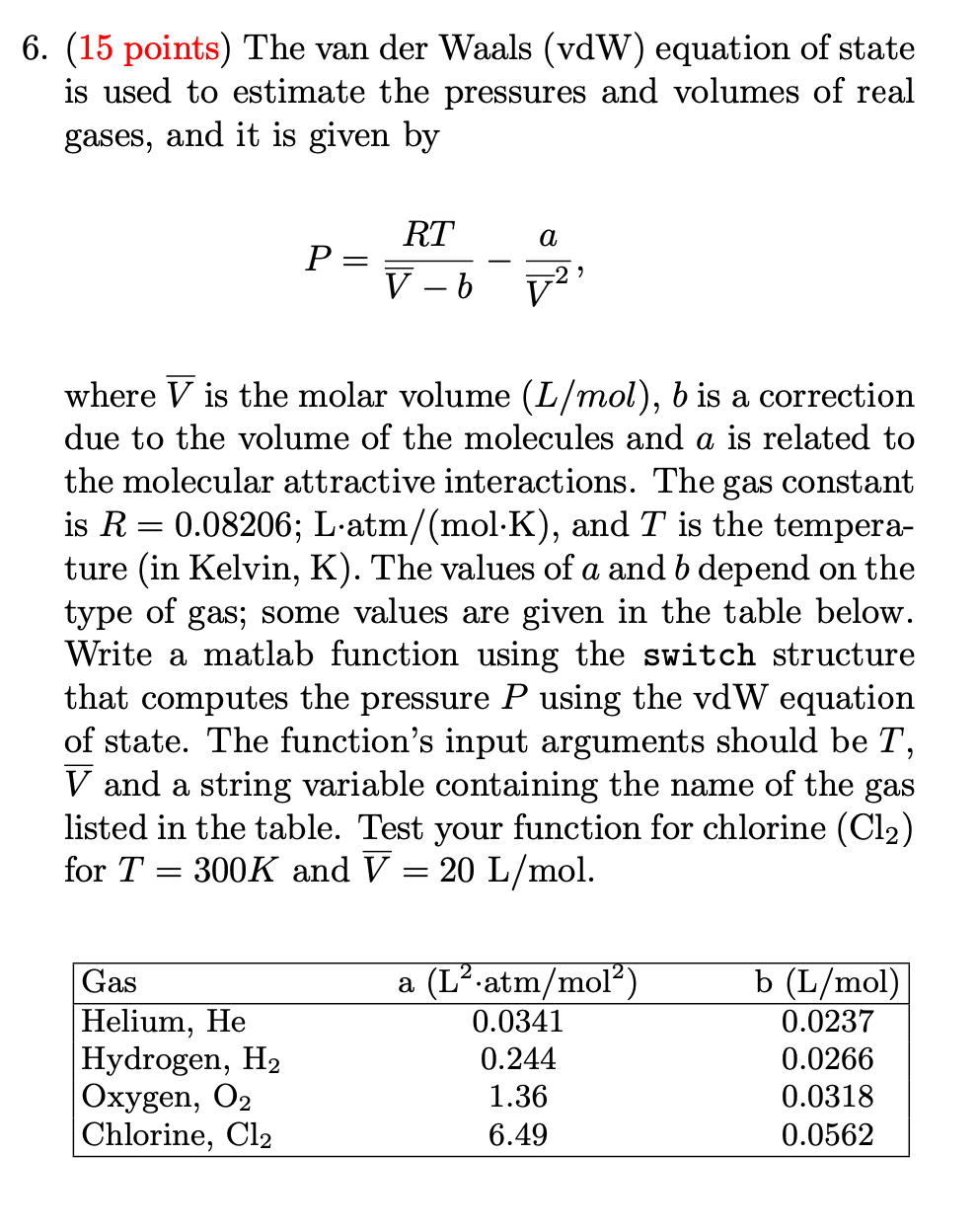
Question: Matlab code pls 6. (15 points) The van der Waals (vdW) equation of state is used to estimate the pressures and volumes of real gases,
 Matlab code pls
Matlab code pls
6. (15 points) The van der Waals (vdW) equation of state is used to estimate the pressures and volumes of real gases, and it is given by a RT P= V b = V = where V is the molar volume (L/mol), b is a correction due to the volume of the molecules and a is related to the molecular attractive interactions. The gas constant is R=0.08206; L'atm/(mol.K), and T is the tempera- ture (in Kelvin, K). The values of a and b depend on the type of gas; some values are given in the table below. Write a matlab function using the switch structure that computes the pressure P using the vdW equation of state. The function's input arguments should be T, V and a string variable containing the name of the gas listed in the table. Test your function for chlorine (C12) for T 300K and V = 20 L/mol. = = Gas Helium, He Hydrogen, H2 Oxygen, O2 Chlorine, Cl2 a (L'.atm/mol) 0.0341 0.244 1.36 6.49 b (L/mol) 0.0237 0.0266 0.0318 0.0562
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


