Question: matlab code with explanation 3. The ideal gas equation relates the volume, pressure, temperature, and the quantity of a y: nRT Where V is the
matlab code with explanation
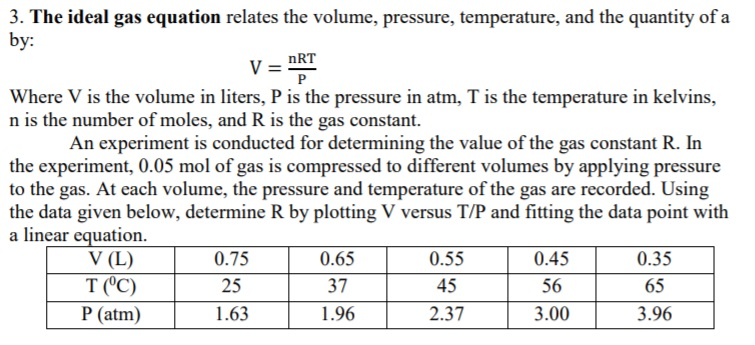
3. The ideal gas equation relates the volume, pressure, temperature, and the quantity of a y: nRT Where V is the volume in liters, P is the pressure in atm, T is the temperature in kelvins, n is the number of moles, and R is the gas constant. An experiment is conducted for determining the value of the gas constant R. In the experiment, 0.05 mol of gas is compressed to different volumes by applying pressure to the gas. At each volume, the pressure and temperature of the gas are recorded. Using the data given below, determine R by plotting V versus T/P and fitting the data point with a linear equation. V (L) T OC) P (atm) 0.75 25 1.63 0.65 37 1.96 0.55 45 2.37 0.45 56 3.00 0.35 65 3.96
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


