Question: May anyone help me with this question? 1. Consider the cubic unit cell of Cu20 (a - 4.27 ): Assign the red and blue ions
May anyone help me with this question?
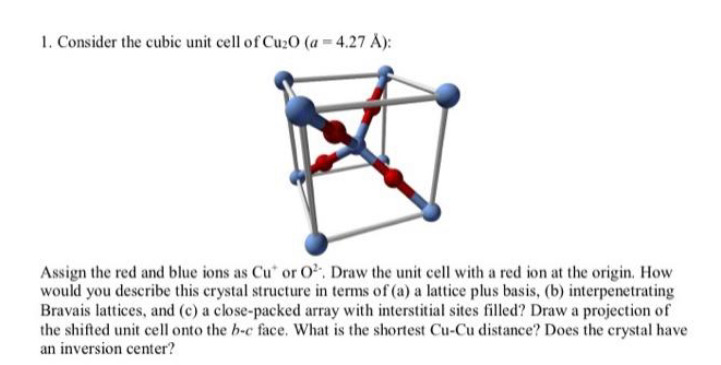
1. Consider the cubic unit cell of Cu20 (a - 4.27 ): Assign the red and blue ions as Cut or O?. Draw the unit cell with a red ion at the origin. How would you describe this crystal structure in terms of (a) a lattice plus basis, (b) interpenetrating Bravais lattices, and (c) a close-packed array with interstitial sites filled? Draw a projection of the shifted unit cell onto the b-c face. What is the shortest Cu-Cu distance? Does the crystal have an inversion center? 1. Consider the cubic unit cell of Cu20 (a - 4.27 ): Assign the red and blue ions as Cut or O?. Draw the unit cell with a red ion at the origin. How would you describe this crystal structure in terms of (a) a lattice plus basis, (b) interpenetrating Bravais lattices, and (c) a close-packed array with interstitial sites filled? Draw a projection of the shifted unit cell onto the b-c face. What is the shortest Cu-Cu distance? Does the crystal have an inversion center
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


