Question: May I get some help with Part D. I am not sure my Ecell Theoretical is correct, and I just don't know how to get
May I get some help with Part D. I am not sure my Ecell Theoretical is correct, and I just don't know how to get the Unknown Theoretical

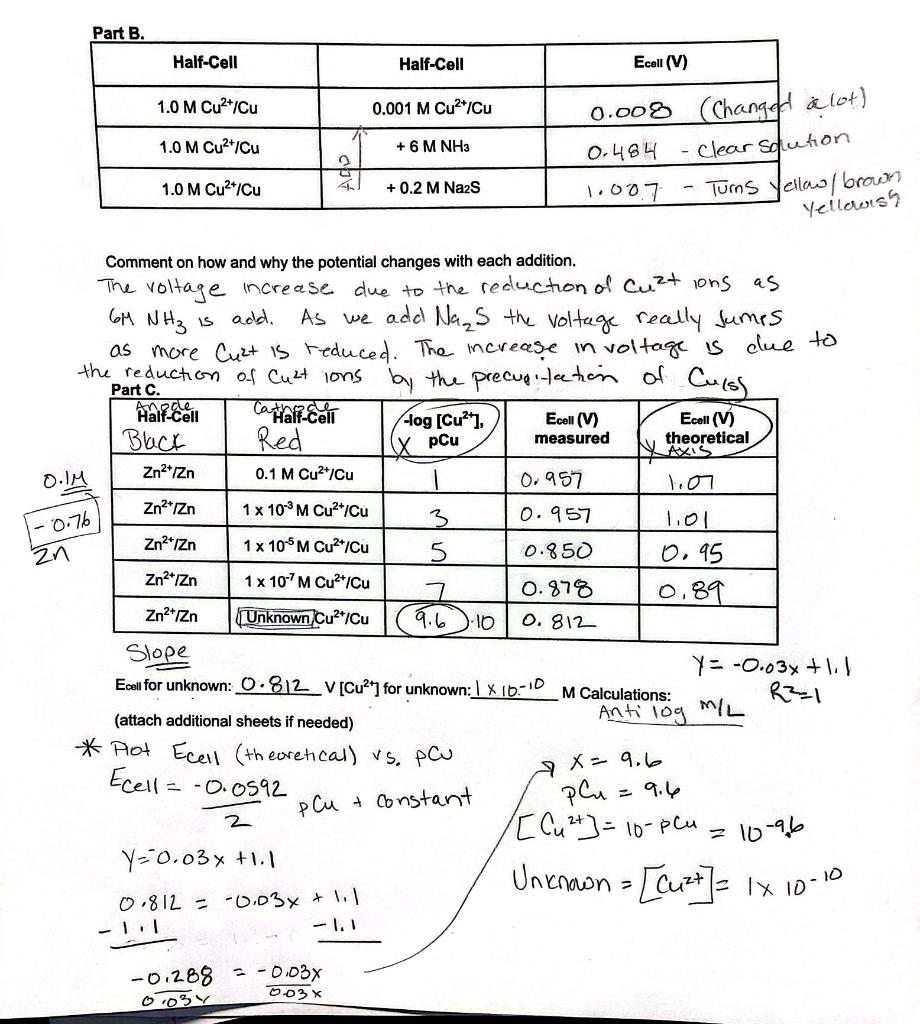
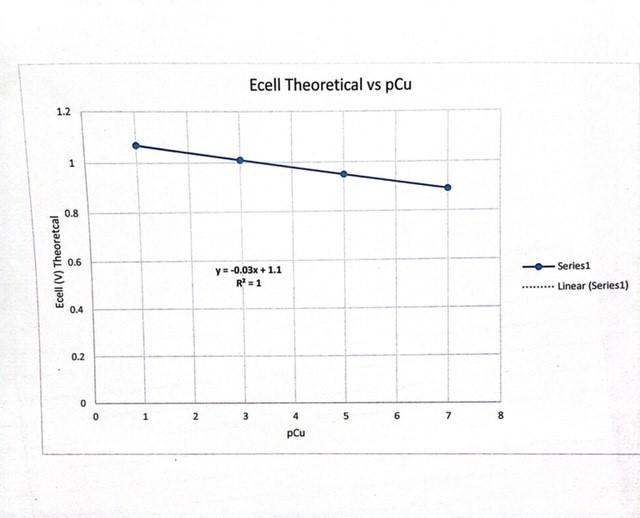
Data Analysis for Part 1. Calculate a theoretical value of Ecell using standard reduction potentials and the Nernst equation. 0.0592 Ecell = Eco log2 n Ecell = Ecell 0.0592 [Zn2+1 -log [Cu2+1 n 2. Calculate pCu. pCu = -log(Cu2+) 3. Plot Ecel Measured and Ecell Calculated vs. pCu using Excel. The equation for the line will take -0.0592 the form of Ecell pCu + constant 2 4. Use the plot to determine the concentration of Cu2+ in the unknown solution. Part B. Half-Cell Half-Cell Ecoll (V) 1.0 M Cu2+/Cu 0.001 M Cu2+ Cu 1.0 M Cu2+Cu + 6 M NH3 0.008 (Change a lot) 0.484 Clear Solution Tums Yellow brown 1.0 M Cu2+/Cu + 0.2 M Na2S - 1.007 Yellowish GM NH is add. W pCu O.IM Zn2+ Izn 1- 0.76 zn Comment on how and why the potential changes with each addition. The voltage increase due to the reduction of Cut ons as As we add Nas the voltage really Jumps as more Cuzt is reduced. The increase in voltage is due to the reduction of Cuctions by the precuci tation of Curss Half-Cell Ca half-celi -log(Cu), Ecell (V) Ecell (V) Black Red measured theoretical Zna Zn 0.1 M Cu2+/Cu 0.957 1.oo 1 x 10' M Cu2+ Cu 3 0.957 Zn2+1Zn 1 x 105 M Cu2+/Cu 5 0.850 0.95 Zn2+iZn 1x 10-7M Cu2+/Cu 0.878 0.89 Zn2+rZn Unknown Cu*Cu 9.6 -10 0.812 Slope Y = -0.03% +1 Ecell for unknown: 0.812_V [Cu247 for unknown: 1 X 10-10 M Calculations: R2=1 Anti log (attach additional sheets if needed) * Plot Ecell (theoretical) vs. pcu Ecella = -0.0592 plu = 9.6 2 [Cu 2+] = 10-plu Y=0.03% +1.1 0.812 = -0.03x + 1 -1.1 MIL 7 x = 9.6 pCu & Constant 2 10-96 Uniknown = [cuzt]= 1x 10-10 -0,288 -0.03% 0.03 0034 Ecell Theoretical vs pCu 1.2 1 0.8 Ecell (V) Theoretcal 0.6 Series1 Y-0.03x + 1.1 R=1 Linear (Series1) 0.4 0.2 0 1 2 3 4 5 6 7 8 pCu
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


