Question: * * * Measuring the diffusion coefficient of dissolved gases in liquids. * * * The diffusivity of dissolved gases, especially O 2 , in
Measuring the diffusion coefficient of dissolved gases in liquids.
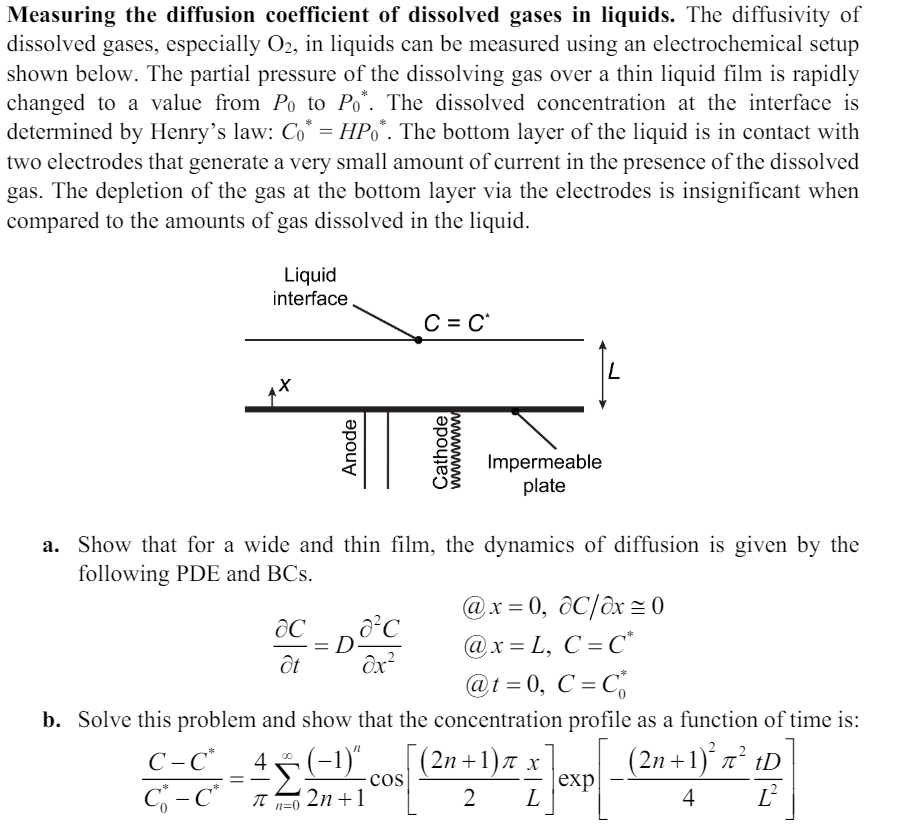
The diffusivity of dissolved gases, especially in liquids can be measured using an electrochemical setup shown below. The partial pressure of the dissolving gas over a thin liquid film is rapidly changed to a value from to The dissolved concentration at the interface is determined by Henry's law: The bottom layer of the liquid is in contact with two electrodes that generate a very small amount of current in the presence of the dissolved gas. The depletion of the gas at the bottom layer via the electrodes is insignificant when compared to the amounts of gas dissolved in the liquid.
a Show that for a wide and thin film, the dynamics of diffusion is given by the
following PDE and BCs
@ delelx~
@
@
b Solve this problem and show that the concentration profile as a function of time is:
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


