Question: MEEN 2 2 3 HW # 3 - Ch 5 The diffusion coefficient for C r 3 + in C r 2 O 3 is
MEEN HW # Ch
The diffusion coefficient for in is at and
at Calculate:
a the activation energy, and
b the constant
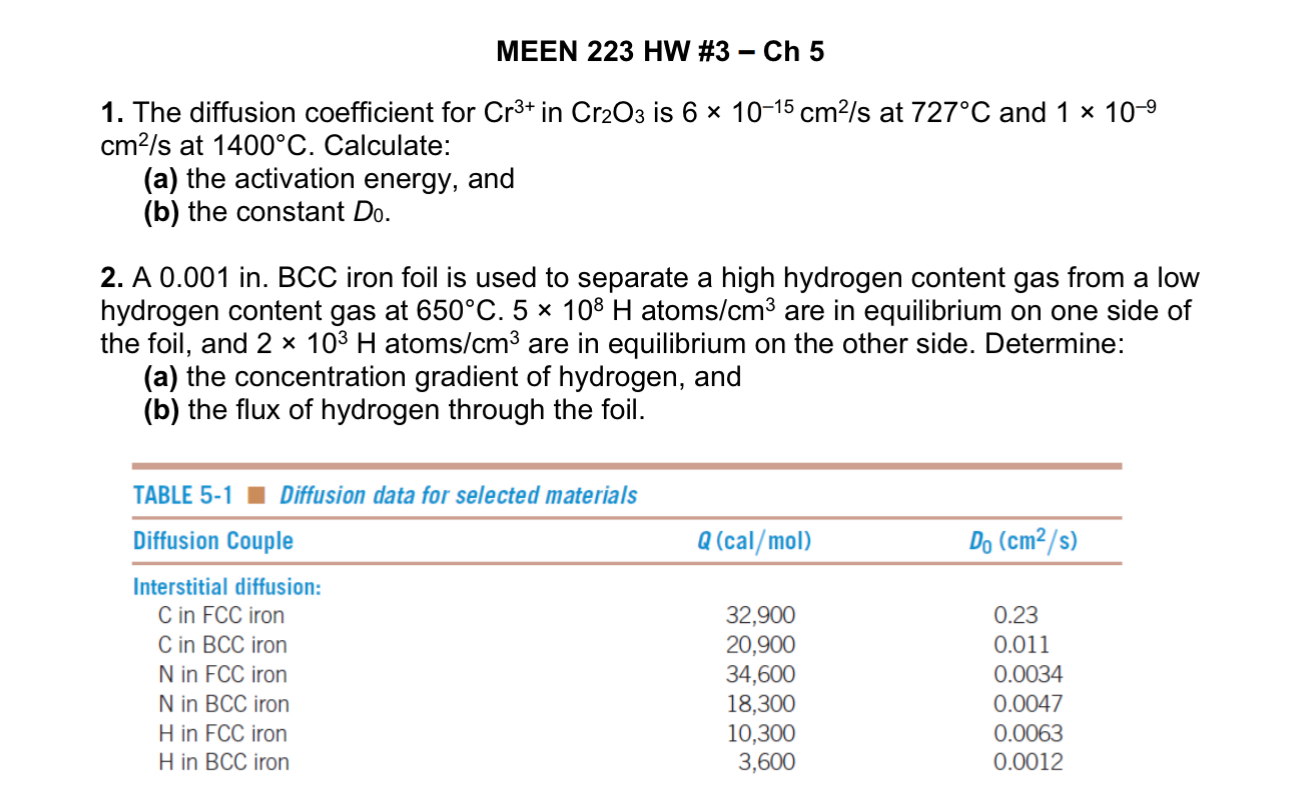
A in BCC iron foil is used to separate a high hydrogen content gas from a low
hydrogen content gas at atoms are in equilibrium on one side of
the foil, and atoms are in equilibrium on the other side. Determine:
a the concentration gradient of hydrogen, and
b the flux of hydrogen through the foil.MEEN HW # Ch
The diffusion coefficient for in is at and
at Calculate:
a the activation energy, and
b the constant
A in BCC iron foil is used to separate a high hydrogen content gas from a low
hydrogen content gas at atoms are in equilibrium on one side of
the foil, and atoms are in equilibrium on the other side. Determine:
a the concentration gradient of hydrogen, and
b the flux of hydrogen through the foil.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


