Question: Mendeleev wrote chemical formulas using superscripts to indicate the number of atoms of each element in the compound. Modern practice is to reserve superscripts for
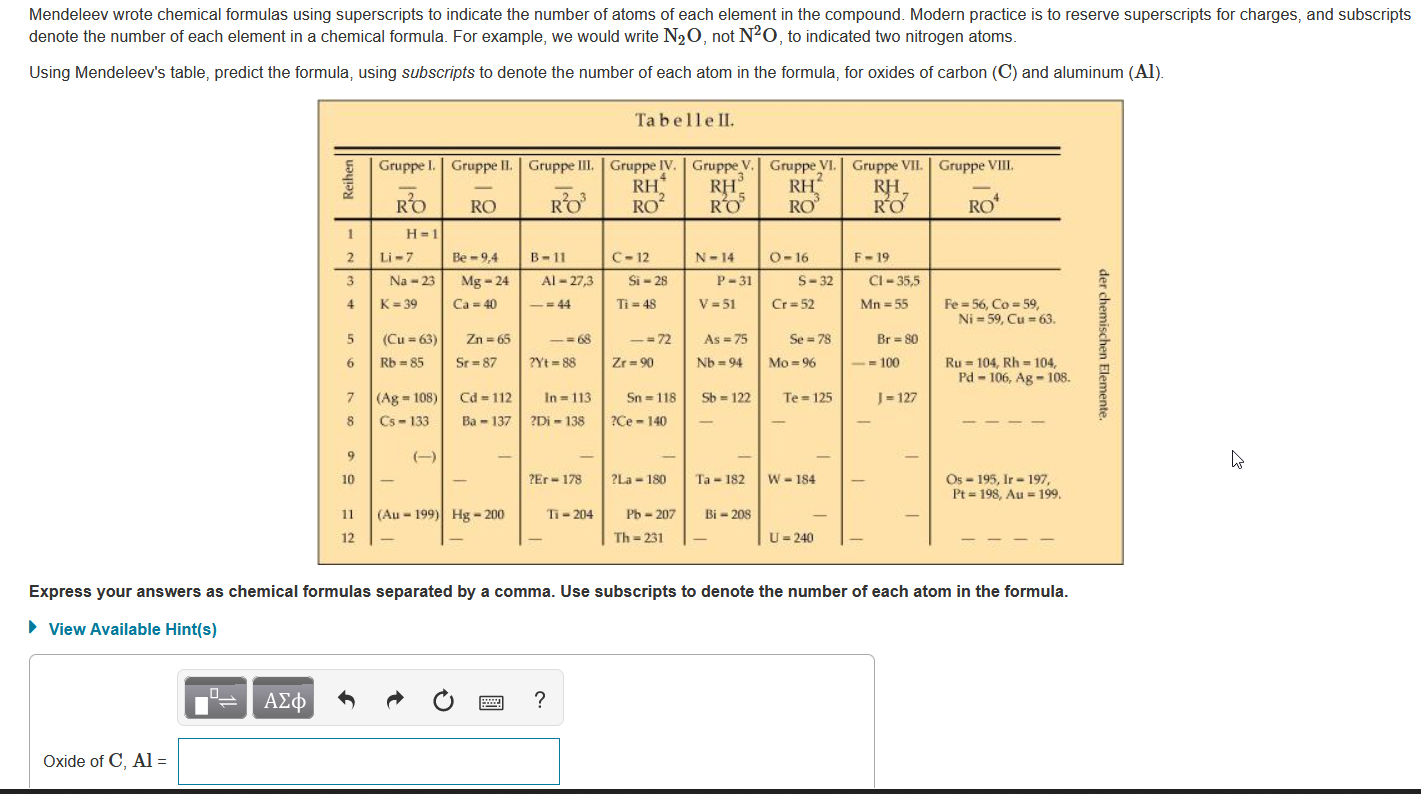
Mendeleev wrote chemical formulas using superscripts to indicate the number of atoms of each element in the compound. Modern practice is to reserve superscripts for charges, and subscripts denote the number of each element in a chemical formula. For example, we would write N20, not N, to indicated two nitrogen atoms. Using Mendeleev's table, predict the formula, using subscripts to denote the number of each atom in the formula, for oxides of carbon (C) and aluminum (Al). Ta belle II. 1 2 3 Gruppe 1. Gruppe II. Gruppe III. Gruppe IV. | Gruppe V. Gruppe VI. Gruppe VII. Gruppe VIII. RH RH RH RH RO RO RO RO RO RO RO RO H-1 Li-7 Be -9,4 B-11 C-12 N-14 0-16 F-19 Na - 23 Mg - 24 Al-27,3 Si - 28 P-31 S-32 CI-35,5 K=39 Ca = 40 = 44 Ti = 48 Cr=52 Mn = 55 Fe = 56, Co=59 Ni = 59, Cu-63 (Cu = 63) Zn = 65 -= 68 - = 72 As = 75 Se = 78 Br = 80 Rb = 85 Sr=87 2Yt=88 Zr = 90 Nb=94 Mo=96 -=100 Ru = 104, Rh = 104, Pd - 106, Ag - 108 (Ag - 108) Cd-112 In = 113 Sn = 118 Sb = 122 Te = 125 J = 127 Cs-133 Ba - 137 ?Di - 138 ?Ce - 140 4 V = 51 5 der chemischen Elemente, 6 7 8 - 9 (-) - 10 ?Er-178 ?La-180 Ta-182 W-184 Os - 195, Ir - 197, Pt = 198, Au = 199 11 (Au-199) Hg - 200 Ti - 204 Bi - 208 Pb - 207 Th=231 12 U-240 - Express your answers as chemical formulas separated by a comma. Use subscripts to denote the number of each atom in the formula. View Available Hint(s) ? Oxide of C, Al =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


