Question: Methane (CH4) is burned with dry air in a continuous steady-state combustion reactor to yield a mixture of carbon monoxide, carbon dioxide and water. The
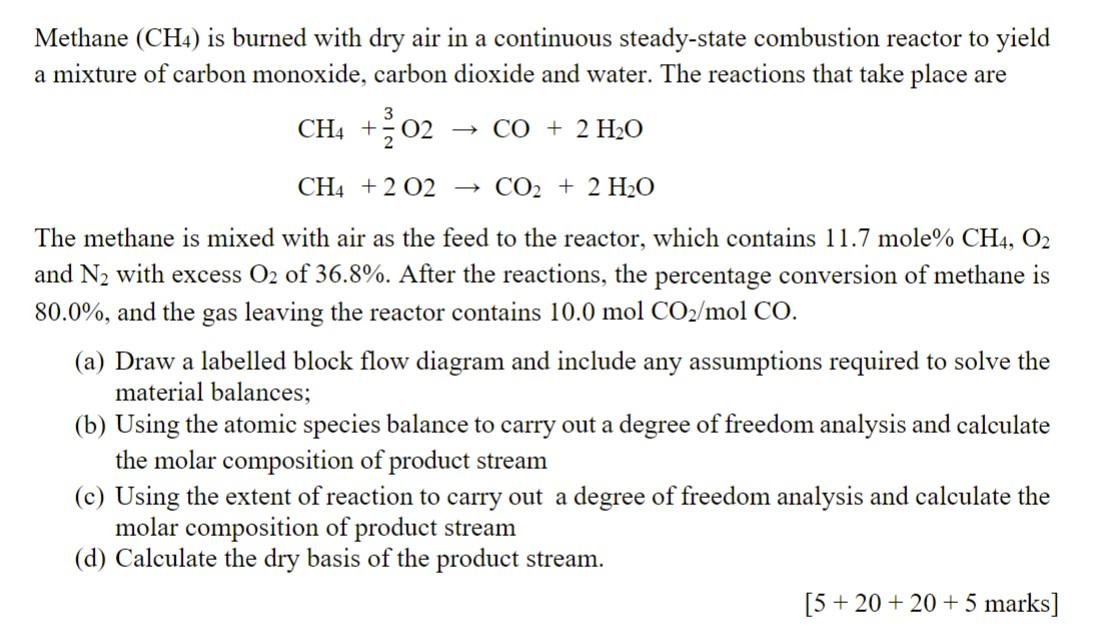
Methane (CH4) is burned with dry air in a continuous steady-state combustion reactor to yield a mixture of carbon monoxide, carbon dioxide and water. The reactions that take place are CH4+23O2CO+2H2OCH4+2O2CO2+2H2O The methane is mixed with air as the feed to the reactor, which contains 11.7mole%CH4,O2 and N2 with excess O2 of 36.8%. After the reactions, the percentage conversion of methane is 80.0%, and the gas leaving the reactor contains 10.0molCO2/molCO. (a) Draw a labelled block flow diagram and include any assumptions required to solve the material balances; (b) Using the atomic species balance to carry out a degree of freedom analysis and calculate the molar composition of product stream (c) Using the extent of reaction to carry out a degree of freedom analysis and calculate the molar composition of product stream (d) Calculate the dry basis of the product stream. [5+20+20+5marks]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


