Question: Methane is burned with 20% excess air; both methane and air being at 298 K. The standard heat of combustion of methane at 298
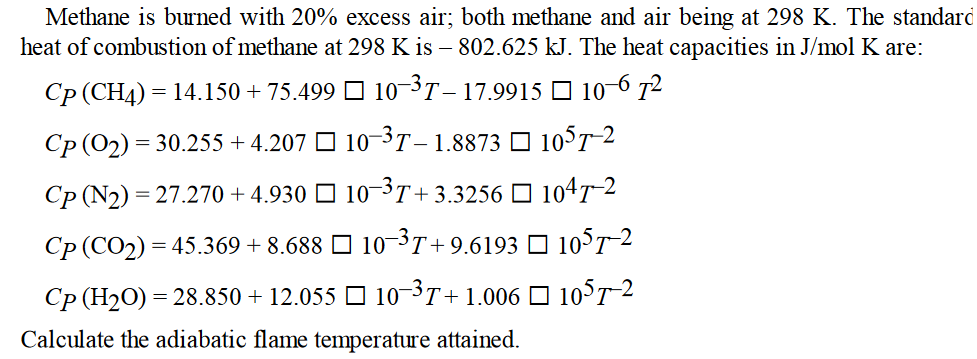
Methane is burned with 20% excess air; both methane and air being at 298 K. The standard heat of combustion of methane at 298 K is 802.625 kJ. The heat capacities in J/mol K are: Cp (CH4) = 14.150 +75.499 10-T 17.9915 | 106 7 Cp(O2)=30.255+4.207 L 10-3T 1.8873 L 105T-2 Cp(N2)=27.270+4.930 L 10-3T+3.3256 L 104T-2 Cp (CO2)=45.369 +8.688 L] 10-3T+9.6193 L 105T-2 Cp (HO) = 28.850 +12.055 10-T+1.006 105T-2 Calculate the adiabatic flame temperature attained.
Step by Step Solution
3.46 Rating (153 Votes )
There are 3 Steps involved in it
The detailed answer for the above questio... View full answer

Get step-by-step solutions from verified subject matter experts


