Question: Methane is produced by Archaea growing on either hydrogen (H2) or acetate (CH3COOH) as electron donors. a. Neglecting cell growth, which is very small, write
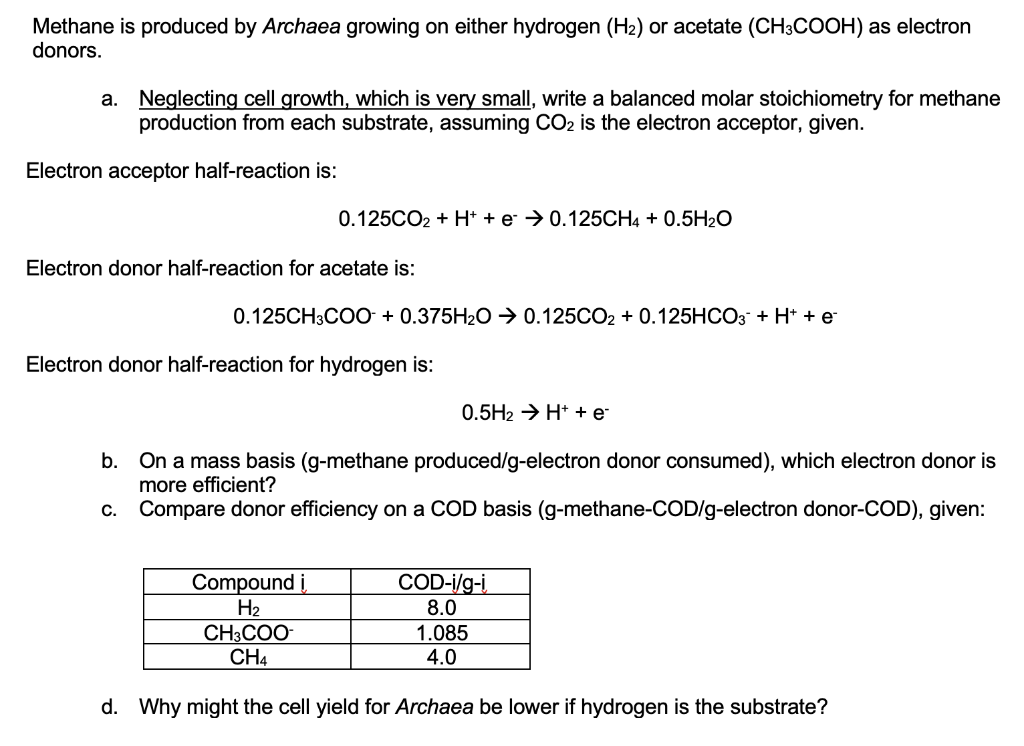
Methane is produced by Archaea growing on either hydrogen (H2) or acetate (CH3COOH) as electron donors. a. Neglecting cell growth, which is very small, write a balanced molar stoichiometry for methane production from each substrate, assuming CO2 is the electron acceptor, given. Electron acceptor half-reaction is: 0.125CO2 + H+ + e + 0.125CH4 + 0.5H2O Electron donor half-reaction for acetate is: 0.125CH3COO + 0.375H20 0.125CO2 + 0.125HCO3 + H+ + e Electron donor half-reaction for hydrogen is: 0.5H2 H+ + e b. On a mass basis (g-methane produced/g-electron donor consumed), which electron donor is more efficient? C. Compare donor efficiency on a COD basis (g-methane-COD/g-electron donor-COD), given: Compound H2 CH3COO CH4 COD-i/g-i 8.0 1.085 4.0 d. Why might the cell yield for Archaea be lower if hydrogen is the substrate
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


