Question: Methyl iodide can be produced by reacting hydro - iodic acid with an excess methanol via the reaction: H I + C H 3 O
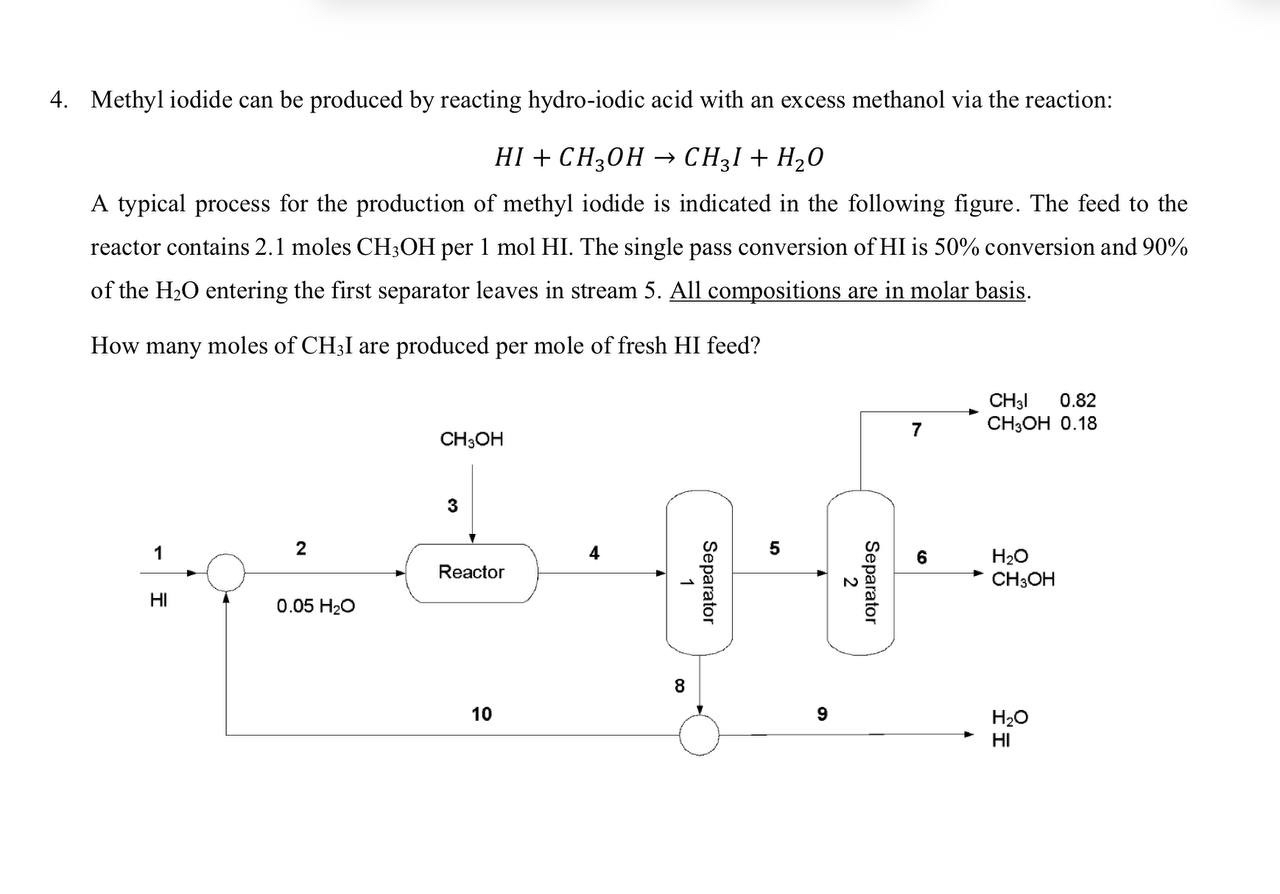
Methyl iodide can be produced by reacting hydroiodic acid with an excess methanol via the reaction:
A typical process for the production of methyl iodide is indicated in the following figure. The feed to the reactor contains moles per molHI. The single pass conversion of is conversion and of the entering the first separator leaves in stream All compositions are in molar basis.
How many moles of I are produced per mole of fresh feed?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


