Question: MISSED THIS? Read Section 20.6 (Pages 917922 ) . An electrochemical cell is based on the following two half-reactions: Oxidation: Pb(s)Pb2+(aq,0.16M)+2e, Reduction: MnO4(aq,1.35M)+4H+(aq12.2M)+3eMnO2(s)+2H2O(1),E=1.68V Part A
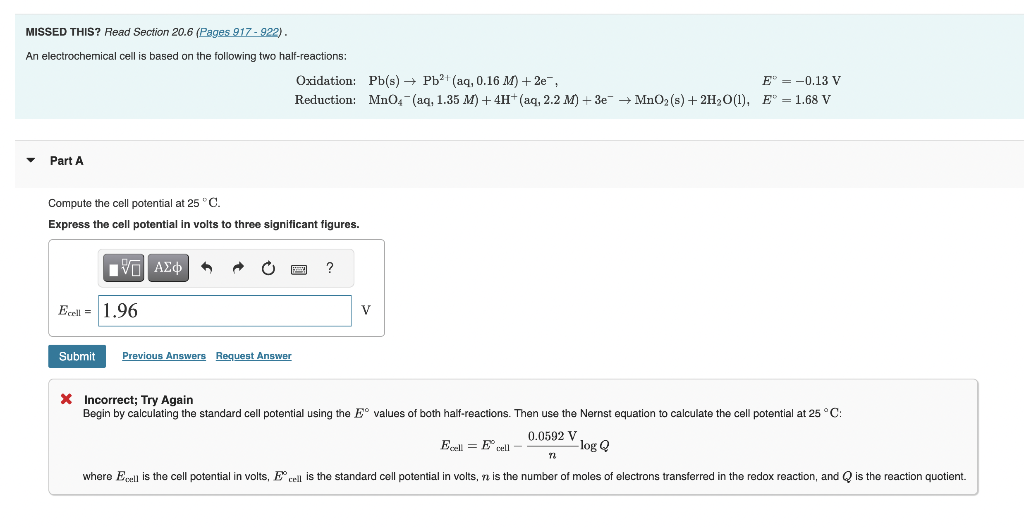
MISSED THIS? Read Section 20.6 (Pages 917922 ) . An electrochemical cell is based on the following two half-reactions: Oxidation: Pb(s)Pb2+(aq,0.16M)+2e, Reduction: MnO4(aq,1.35M)+4H+(aq12.2M)+3eMnO2(s)+2H2O(1),E=1.68V Part A Compute the cell potential at 25C. Express the cell potential in volts to three significant figures. * Incorrect; Try Again Begin by calculating the standard cell potential using the E values of both half-reactions. Then use the Nernst equation to calculate the cell potential at 25C : Ecell=Ecelln0.0592Vlog
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


