Question: MISSED THIS? Read Section 5.3 (Pages 173 - 174) ; Watch MWE 5.4. Consider the following precipitation reaction: 2Na3PO4(aq)+3CuCl2(aq)Cu3(PO4)2(s)+6NaCl(aq) What volume of 0.182MNNa3PO4 solution is
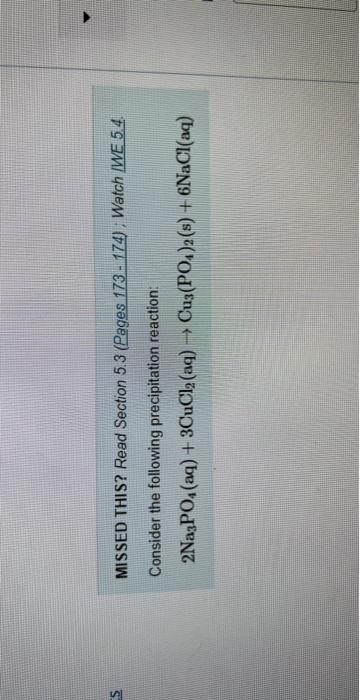
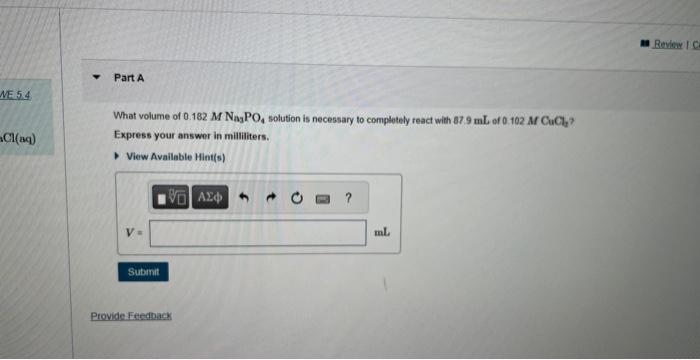
MISSED THIS? Read Section 5.3 (Pages 173 - 174) ; Watch MWE 5.4. Consider the following precipitation reaction: 2Na3PO4(aq)+3CuCl2(aq)Cu3(PO4)2(s)+6NaCl(aq) What volume of 0.182MNNa3PO4 solution is necessary to complotely react with 87.9mL of 0.102MCuCl7 ? Express your answer in milliliters
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


