Question: MISSED THIS? Read Section 7.8 (Rages.296 - 289) : Watch WE 7.9. Calculale Hrs for the following reaction: CH4(g)+4Cl2(g)CCl4(g)+4HCl(g) given these reactions and their H
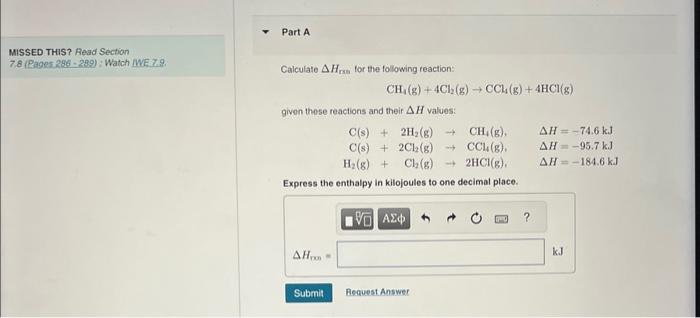
MISSED THIS? Read Section 7.8 (Rages.296 - 289) : Watch WE 7.9. Calculale Hrs for the following reaction: CH4(g)+4Cl2(g)CCl4(g)+4HCl(g) given these reactions and their H values: C(s)+2H2(g)CH4(g).H=74.6kJC(s)+2Cl2(g)CCl4(g),H=95.7k.JH2(g)+Cl2(g)2HCl(g),H=184.6k.J Express the enthalpy in kilojoules to one decimal place
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


