Question: MISSED THIS? Watch KCV 8.2; Read Section 8.3. Part B You can click on the Review link to access the section in your eText. For
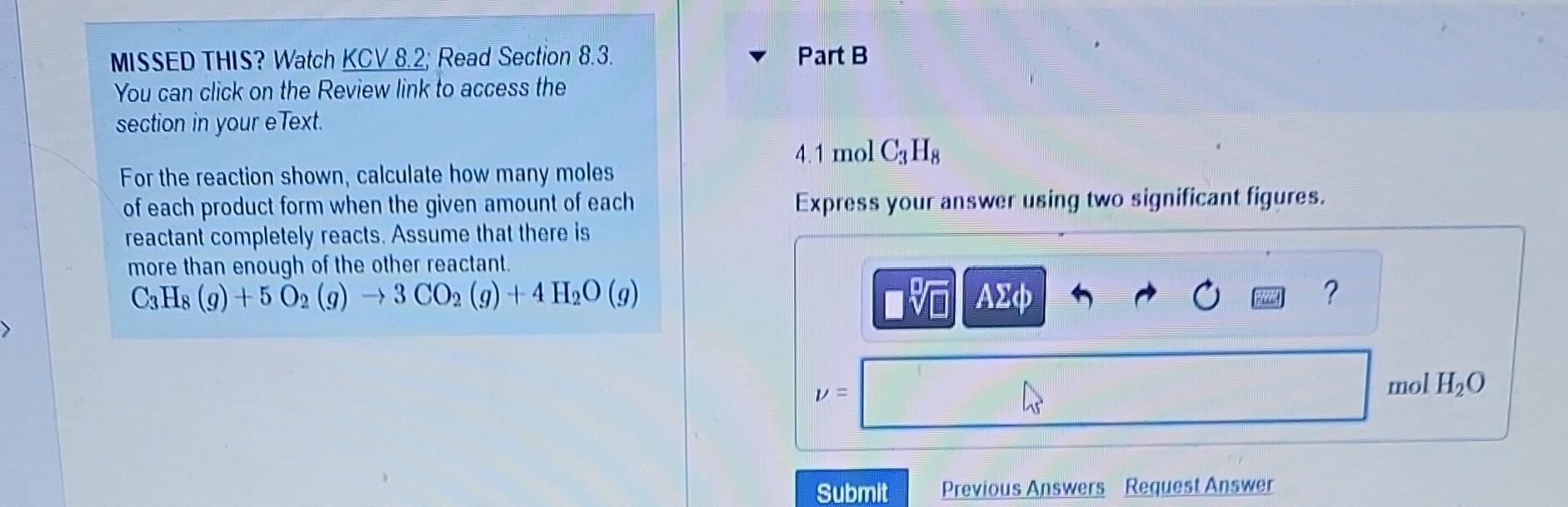
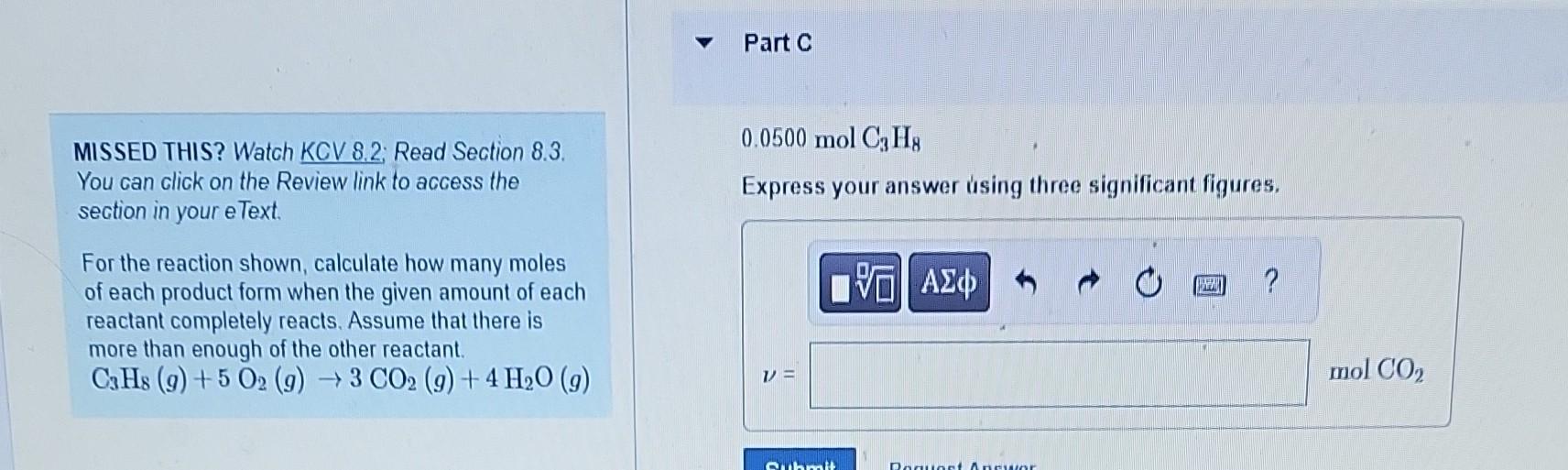
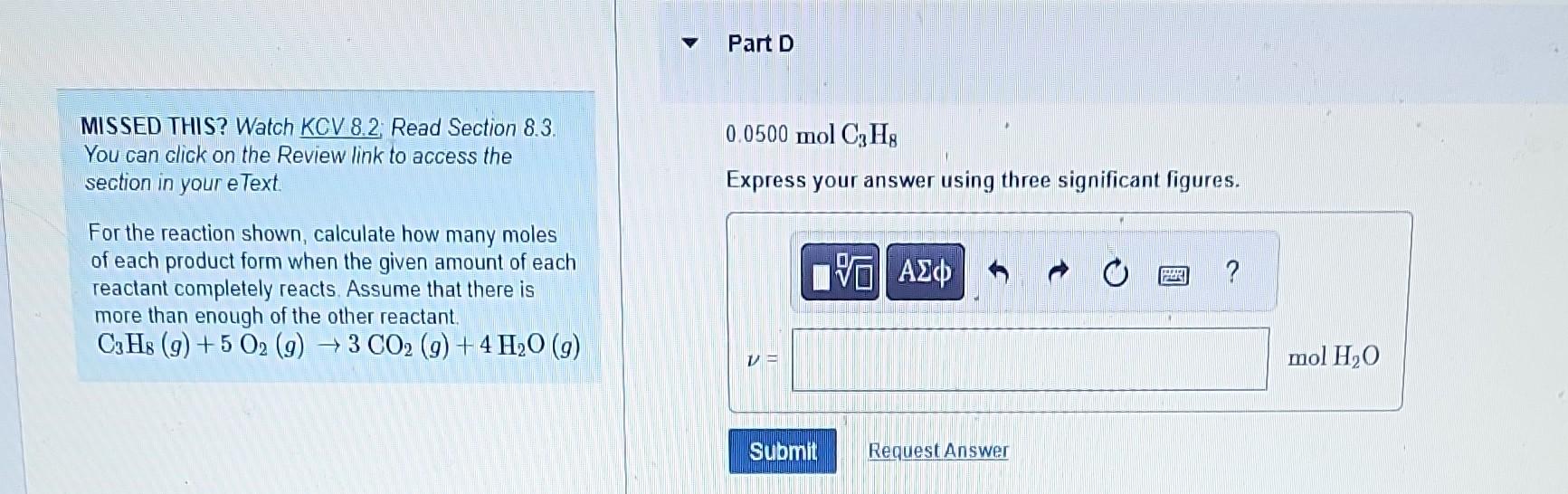
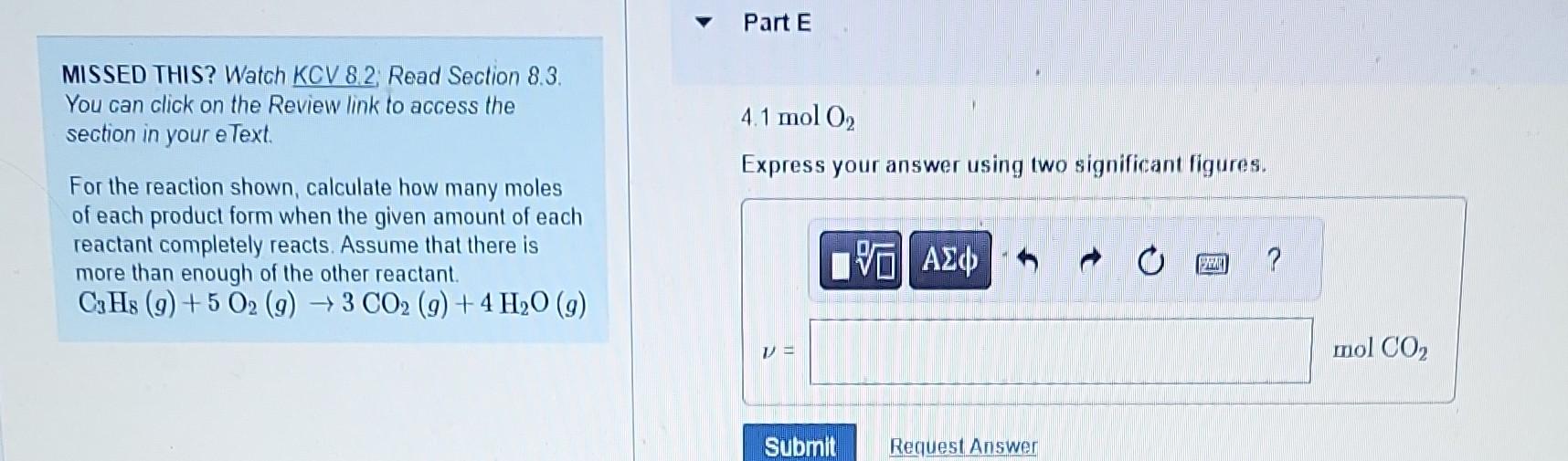
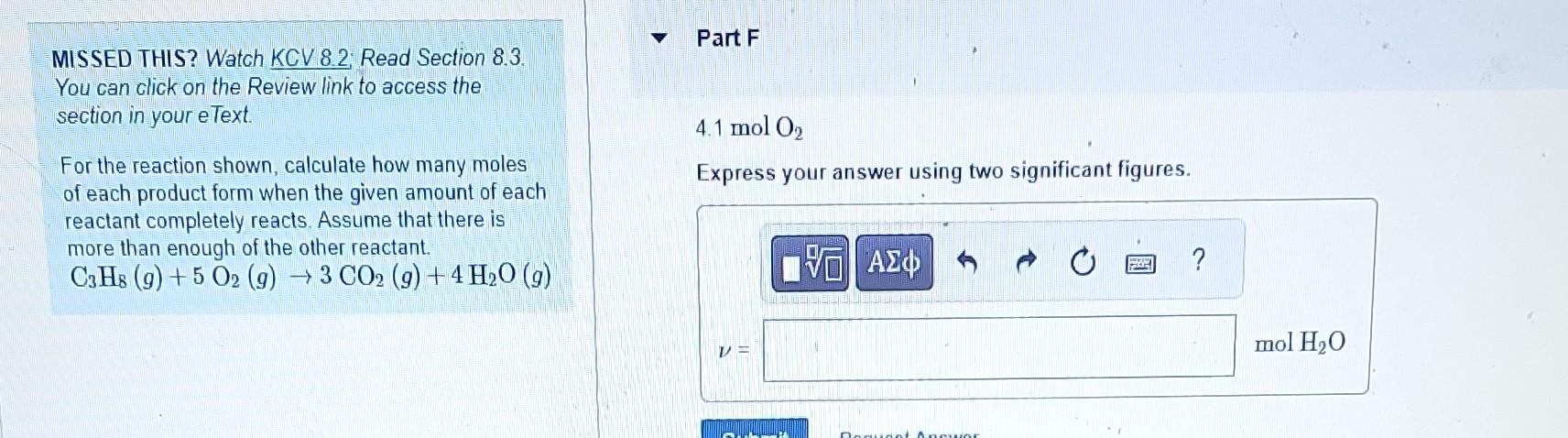
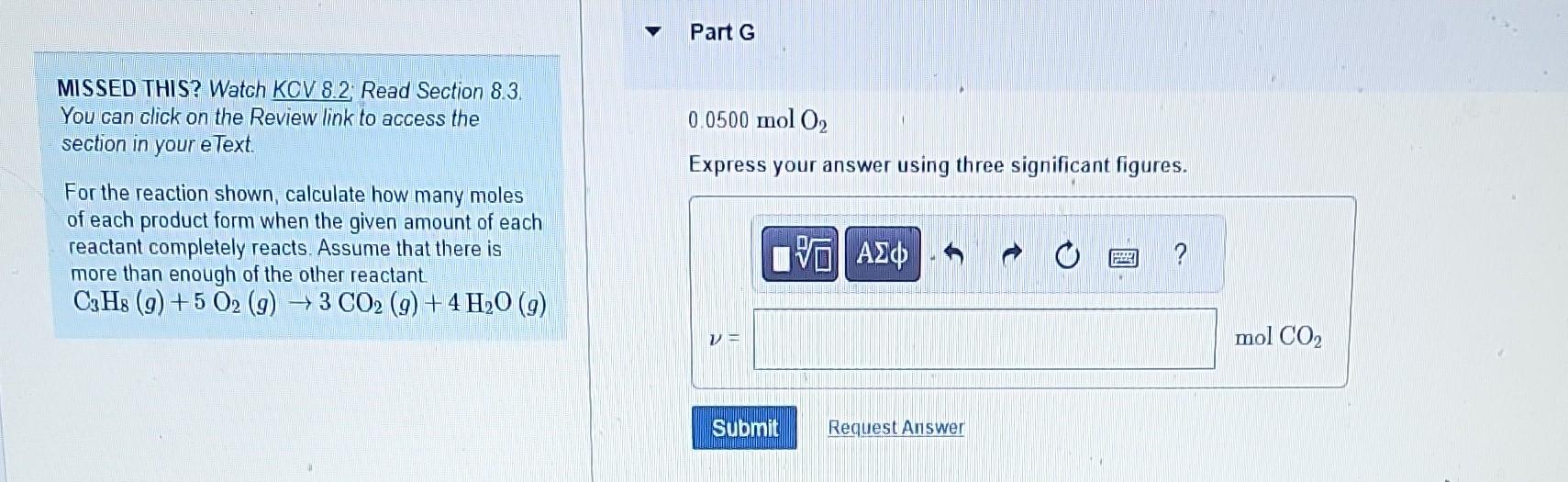
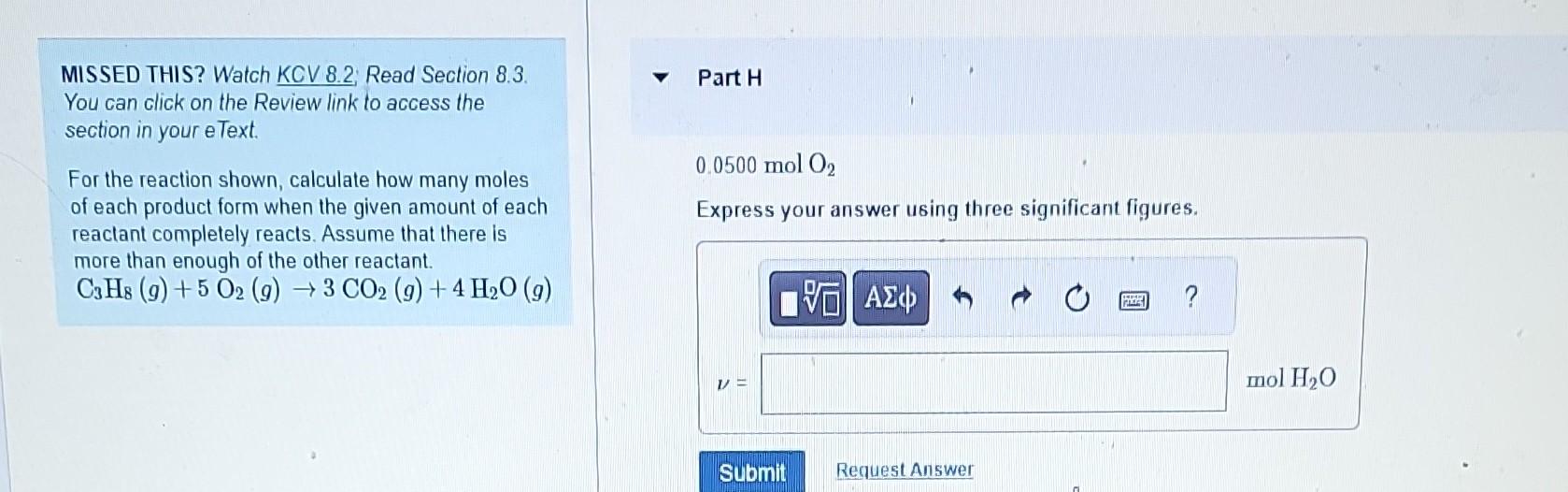
MISSED THIS? Watch KCV 8.2; Read Section 8.3. Part B You can click on the Review link to access the section in your eText. For the reaction shown, calculate how many moles 4.1molC3H8 of each product form when the given amount of each Express your answer using two significant figures. reactant completely reacts. Assume that there is more than enough of the other reactant. C3H8(g)+5O2(g)3CO2(g)+4H2O(g) MISSED THIS? Watch KCV 8.2; Read Section 8.3. 0.0500molC3H8 You can click on the Review link to access the Express your answer using three significant figures. section in your eText. For the reaction shown, calculate how many moles of each product form when the given amount of each reactant completely reacts. Assume that there is more than enough of the other reactant. C3H8(g)+5O2(g)3CO2(g)+4H2O(g) MISSED THIS? Watch KCV 8.2. Read Section 8.3. You can click on the Review link to access the 0.0500molC3H8 section in your eText. Express your answer using three significant figures. For the reaction shown, calculate how many moles of each product form when the given amount of each reactant completely reacts. Assume that there is more than enough of the other reactant. C3H8(g)+5O2(g)3CO2(g)+4H2O(g) MISSED THIS? Watch KCV 8.2: Read Section 8.3. You can click on the Review link to access the section in your eText. 4.1 molO2 For the reaction shown, calculate how many moles of each product form when the given amount of each reactant completely reacts. Assume that there is more than enough of the other reactant. C3H8(g)+5O2(g)3CO2(g)+4H2O(g) MISSED THIS? Watch KCV 8.2 Read Section 8.3. Part F You can click on the Review link to access the section in your eText. 4.1molO2 For the reaction shown, calculate how many moles of each product form when the given amount of each reactant completely reacts. Assume that there is more than enough of the other reactant. C3H8(g)+5O2(g)3CO2(g)+4H2O(g) Express your answer using two significant figures. MISSED THIS? Watch KCV 8.2 Read Section 8.3. You can click on the Review link to access the 0.0500molO2 section in your eText. Express your answer using three significant figures. For the reaction shown, calculate how many moles of each product form when the given amount of each reactant completely reacts. Assume that there is more than enough of the other reactant C3H8(g)+5O2(g)3CO2(g)+4H2O(g) MISSED THIS? Watch KCV 8.2; Read Section 8.3. Part H You can click on the Review link to access the section in your eText. For the reaction shown, calculate how many moles of each product form when the given amount of each reactant completely reacts. Assume that there is more than enough of the other reactant. C3H8(g)+5O2(g)3CO2(g)+4H2O(g)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


