Question: MISSED THIS? Watch KCV: Finding Equilibrium Concentrations from Initial Concentrations IWE: Finding Equilibrium Concentrations from Initial Concentrations and the Equilibrium Constant ; Read Section 16.8.

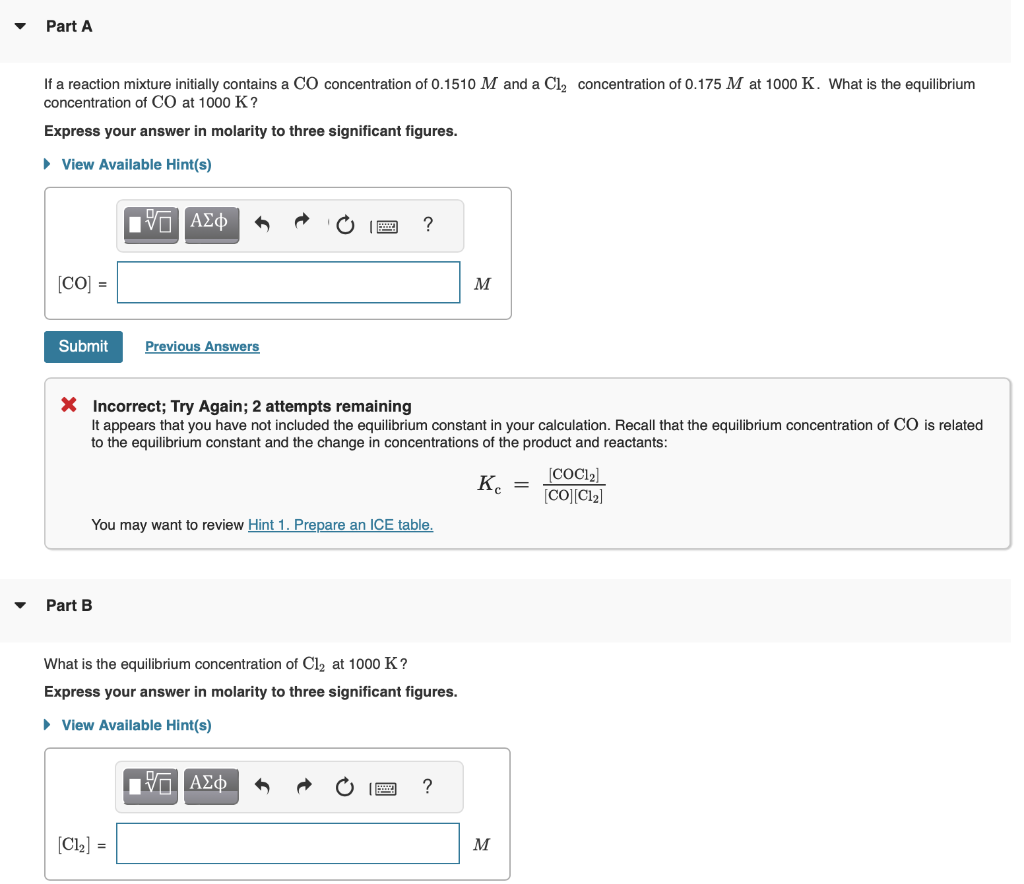
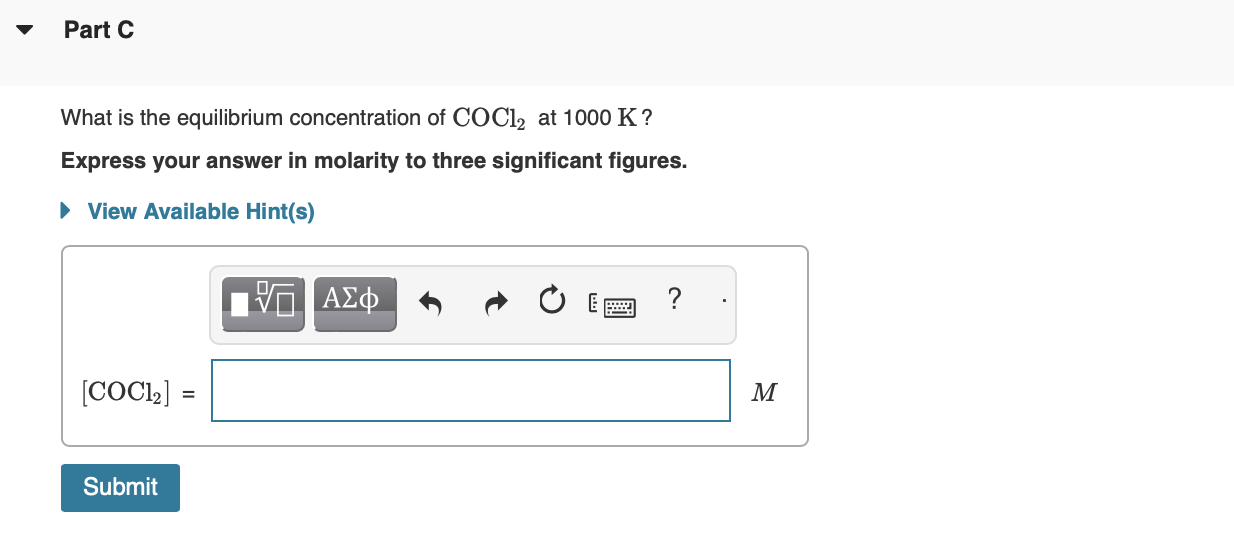
MISSED THIS? Watch KCV: Finding Equilibrium Concentrations from Initial Concentrations IWE: Finding Equilibrium Concentrations from Initial Concentrations and the Equilibrium Constant ; Read Section 16.8. You can click on the Review link to access the section in your eText. For the following reaction, Kc=255 at 1000K. CO(g)+Cl2(g)COCl2(g) If a reaction mixture initially contains a CO concentration of 0.1510M and a Cl2 concentration of 0.175M at 1000K. What is the equilibrium concentration of CO at 1000K ? Express your answer in molarity to three significant figures. X Incorrect; Try Again; 2 attempts remaining It appears that you have not included the equilibrium constant in your calculation. Recall that the equilibrium concentration of CO is related to the equilibrium constant and the change in concentrations of the product and reactants: Kc=[CO][Cl2][COCl2] You may want to review Part B What is the equilibrium concentration of Cl2 at 1000K ? Express your answer in molarity to three significant figures. What is the equilibrium concentration of COCl2 at 1000K ? Express your answer in molarity to three significant figures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


