Question: MnO (A) (Tm = 1785 C) and Nb2Os (B) (Tm = 1520 C) form a binary system in which two compounds exits in the molar
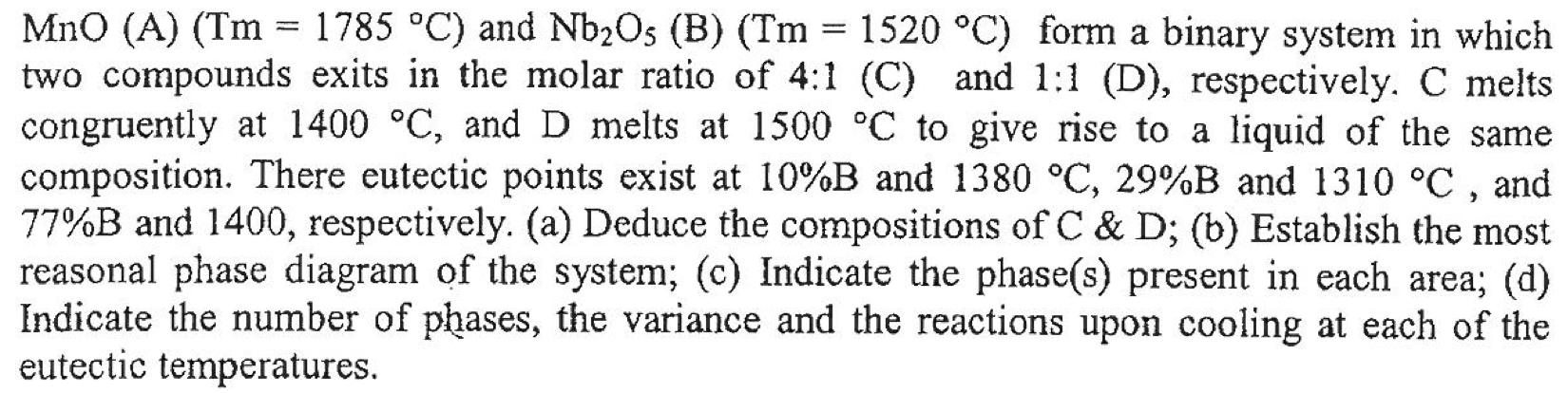
MnO (A) (Tm = 1785 C) and Nb2Os (B) (Tm = 1520 C) form a binary system in which two compounds exits in the molar ratio of 4:1 (C) and 1:1 (D), respectively. C melts congruently at 1400 C, and D melts at 1500 C to give rise to a liquid of the same composition. There eutectic points exist at 10%B and 1380 C, 29%B and 1310 C, and 77%B and 1400, respectively. (a) Deduce the compositions of C & D; (b) Establish the most reasonal phase diagram of the system; (c) Indicate the phase(s) present in each area; (d) Indicate the number of phases, the variance and the reactions upon cooling at each of the eutectic temperatures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


