Question: Model 2: Using Splitting to Solve NMR Structures To solve NMR structures that involve splitting, follow the same steps as before, but add a new
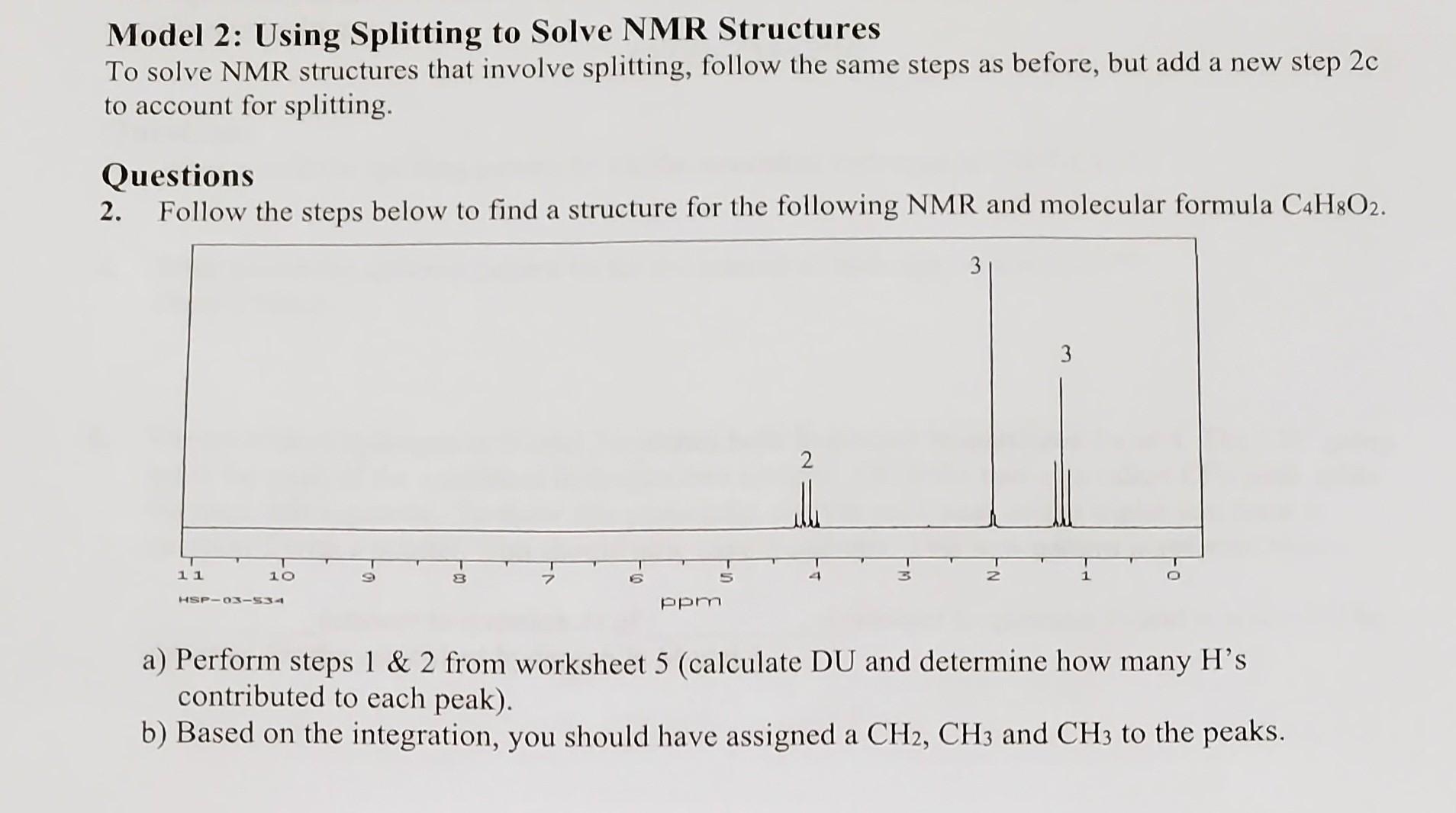

Model 2: Using Splitting to Solve NMR Structures To solve NMR structures that involve splitting, follow the same steps as before, but add a new step 2c to account for splitting. Questions 2. Follow the steps below to find a structure for the following NMR and molecular formula C4H8O2. a) Perform steps 1&2 from worksheet 5 (calculate DU and determine how many H's contributed to each peak). b) Based on the integration, you should have assigned a CH2,CH3 and CH3 to the peaks. c) New step 2c: put groups together based on splitting. i) The 4.2 peak (CH2) is a quartet and has 3 hydrogen neighbors, most likely a CH3.CH2CH3 ii) The 1.3 peak (CH3) is a triplet and has 2 hydrogen neighbors, most likely a CH2.CH3CH2 Since there is only one CH2 group in the molecule the black (i) and blue (ii) CH2 's must be the same. Since the CH2 group is only bonded to one CH3 group (quartet) the red CH3 (i) and the black CH3 must be the same. Splitting indicates there is a CH2CH3 group in the molecule. The peak at 2.1 has no splitting and thus, has no neighboring hydrogen. d) Perform steps 3 \& 4 from worksheet 5 (identify possible functional groups, account for any other carbon). e) Perform step 5. Draw your structure below. f) Does your structure match the molecular formula and chemical shifts
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


