Question: Model 4: Molecules Containing Nitrogen Nitrogen is the only common organic element that makes an odd number of bonds but has an even molecular weight.
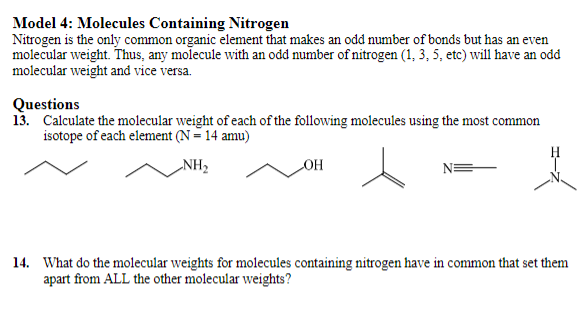
Model 4: Molecules Containing Nitrogen Nitrogen is the only common organic element that makes an odd number of bonds but has an even molecular weight. Thus, any molecule with an odd number of nitrogen (1,3,5, etc) will have an odd molecular weight and vice versa. Questions 13. Calculate the molecular weight of each of the following molecules using the most common isotope of each element (N=14amu) N 14. What do the molecular weights for molecules containing nitrogen have in common that set them apart from ALL the other molecular weights
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


