Question: Module IV. Calculate results and analyze experimental errors Objective. To ansyre and compare experimental emess in the deiorminaton of CO moar volume at STP: Wich
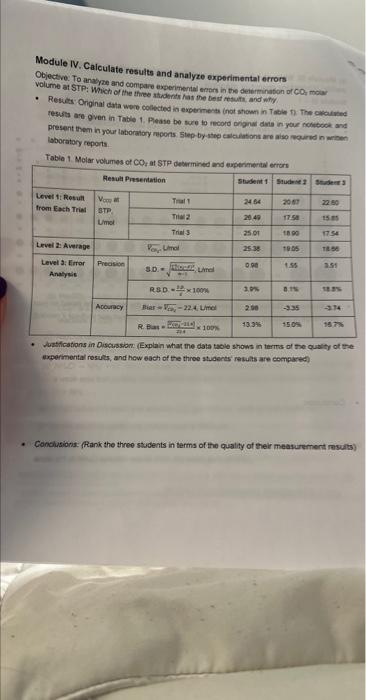
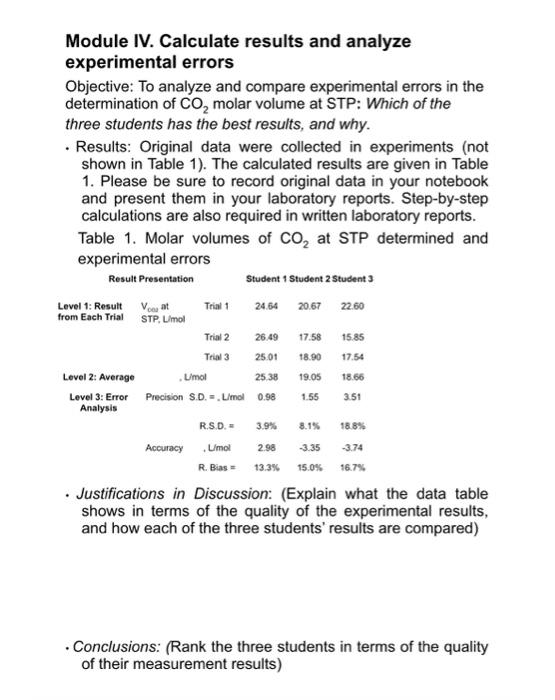
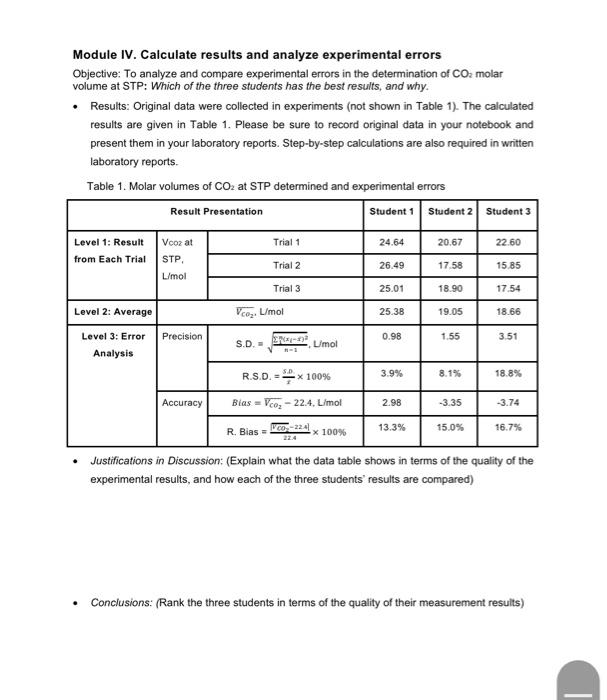
Module IV. Calculate results and analyze experimental errors Objective. To ansyre and compare experimental emess in the deiorminaton of CO moar volume at STP: Wich of the thiee sticderits hat the best resuith, and whily. - Resies: Originat data were colectied is experments inat stown in Tasien in the ovcuived resula are ovan in Table 1. Pleaso bo suce to record ongrim disis in youe notwocok and present them in your laboranory repons 5 tep by step calakitions are aiso wourve in wrben ISboratory ceports. Tabia 1. Molar volumes of COz at STP deburmined and ancerminerial antre - Nestifcotions in Discussiom (Explain what me data taole shows in terris of the qualty of the expermental resils, and how each of the throe stuaerts resuls are compared) - Conctusions: (Ranik the three students in terms of the quality of their measurement results) Module IV. Calculate results and analyze experimental errors Objective: To analyze and compare experimental errors in the determination of CO2 molar volume at STP: Which of the three students has the best results, and why. - Results: Original data were collected in experiments (not shown in Table 1). The calculated results are given in Table 1. Please be sure to record original data in your notebook and present them in your laboratory reports. Step-by-step calculations are also required in written laboratory reports. Table 1. Molar volumes of CO2 at STP determined and experimental errors - Justifications in Discussion: (Explain what the data table shows in terms of the quality of the experimental results, and how each of the three students' results are compared) - Conclusions: (Rank the three students in terms of the quality of their measurement results) Module IV. Calculate results and analyze experimental errors Objective: To analyze and compare experimental errors in the determination of CO2 molar volume at STP: Which of the three students has the best results, and why. - Results: Original data were collected in experiments (not shown in Table 1). The calculated results are given in Table 1. Please be sure to record original data in your notebook and present them in your laboratory reports. Step-by-step calculations are also required in written laboratory reports. Table 1. Molar volumes of CO2 at STP determined and experimental errors - Justifications in Discussion: (Explain what the data table shows in terms of the quality of the experimental results, and how each of the three students' results are compared) - Conclusions: (Rank the three students in terms of the quality of their measurement results) Module IV. Calculate results and analyze experimental errors Objective. To ansyre and compare experimental emess in the deiorminaton of CO moar volume at STP: Wich of the thiee sticderits hat the best resuith, and whily. - Resies: Originat data were colectied is experments inat stown in Tasien in the ovcuived resula are ovan in Table 1. Pleaso bo suce to record ongrim disis in youe notwocok and present them in your laboranory repons 5 tep by step calakitions are aiso wourve in wrben ISboratory ceports. Tabia 1. Molar volumes of COz at STP deburmined and ancerminerial antre - Nestifcotions in Discussiom (Explain what me data taole shows in terris of the qualty of the expermental resils, and how each of the throe stuaerts resuls are compared) - Conctusions: (Ranik the three students in terms of the quality of their measurement results) Module IV. Calculate results and analyze experimental errors Objective: To analyze and compare experimental errors in the determination of CO2 molar volume at STP: Which of the three students has the best results, and why. - Results: Original data were collected in experiments (not shown in Table 1). The calculated results are given in Table 1. Please be sure to record original data in your notebook and present them in your laboratory reports. Step-by-step calculations are also required in written laboratory reports. Table 1. Molar volumes of CO2 at STP determined and experimental errors - Justifications in Discussion: (Explain what the data table shows in terms of the quality of the experimental results, and how each of the three students' results are compared) - Conclusions: (Rank the three students in terms of the quality of their measurement results) Module IV. Calculate results and analyze experimental errors Objective: To analyze and compare experimental errors in the determination of CO2 molar volume at STP: Which of the three students has the best results, and why. - Results: Original data were collected in experiments (not shown in Table 1). The calculated results are given in Table 1. Please be sure to record original data in your notebook and present them in your laboratory reports. Step-by-step calculations are also required in written laboratory reports. Table 1. Molar volumes of CO2 at STP determined and experimental errors - Justifications in Discussion: (Explain what the data table shows in terms of the quality of the experimental results, and how each of the three students' results are compared) - Conclusions: (Rank the three students in terms of the quality of their measurement results)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


