Question: multiple choice question Based on intermolecular forces, which of the following do you expect to be most soluble in waher? ? A CH, OH B
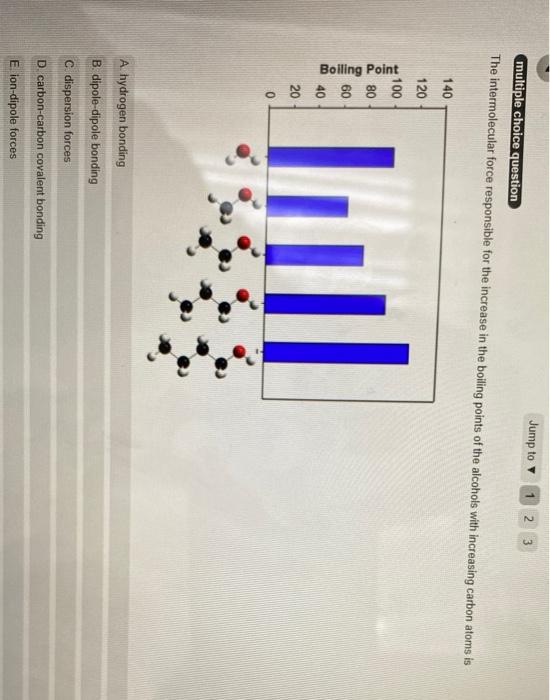
multiple choice question Based on intermolecular forces, which of the following do you expect to be most soluble in waher? ? A CH, OH B CH, C CH D CH,CHOCH,CHE atmotor loin another session Jump to 1 2 3 multiple choice question The intermolecular force responsible for the increase in the boiling points of the alcohols with increasing carbon atoms is 140 120 100 80 Bolling Point 60 il 40 20 0 A hydrogen bonding B. dipole-dipole bonding C dispersion forces D. carbon-carbon covalent bonding E ion-dipole forces
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


