Question: Multiple Choice (Total Point 1.00) #15 Which statement is a logical consequence of the fact that a 0.10 molar solution of potassium acetate, C,H,O, is
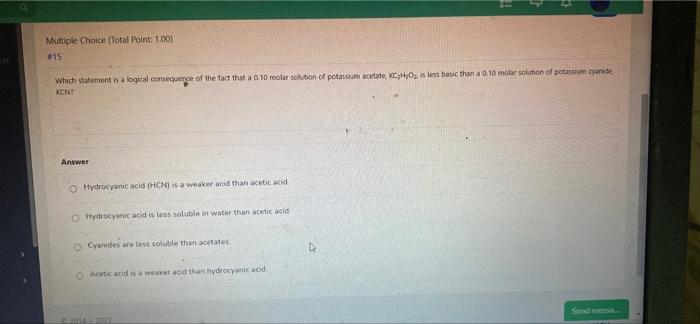
Multiple Choice (Total Point 1.00) #15 Which statement is a logical consequence of the fact that a 0.10 molar solution of potassium acetate, C,H,O, is less basic than a 0.10 molar solution of potassium yanide KONE Answer Hydrocyanic acid (HCN) is a weaker acid than acetic acid Hydrocyanic acid is less soluble in water than acetic acid Cyanides are less soluble than acetate Acades a weak and than tydrocyanic acid Send messa
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


