Question: my work Enter your answer in the provided box. The weak acid HZ has a K, of 2.55 x 10- Calculate the pOH of 0.0250
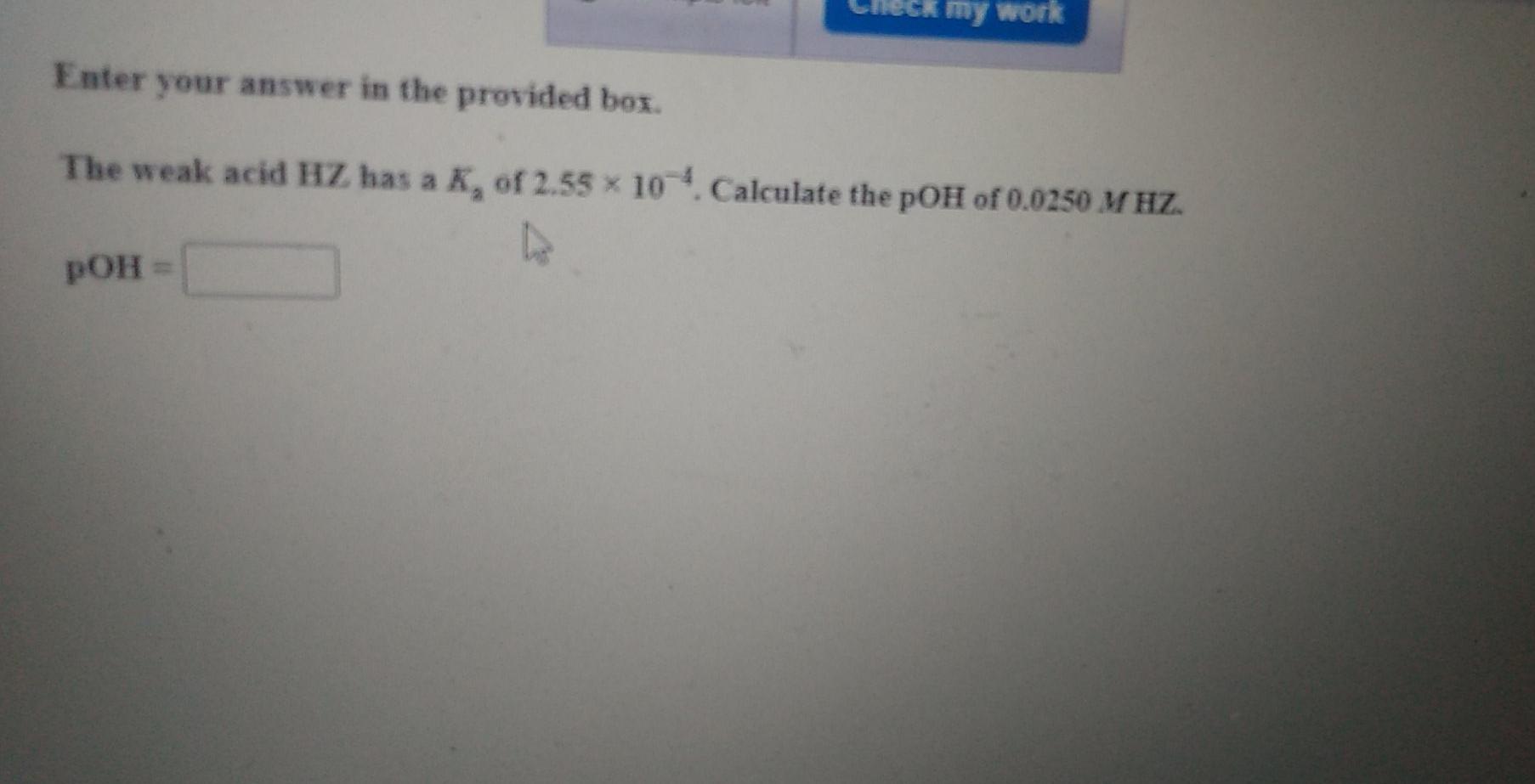
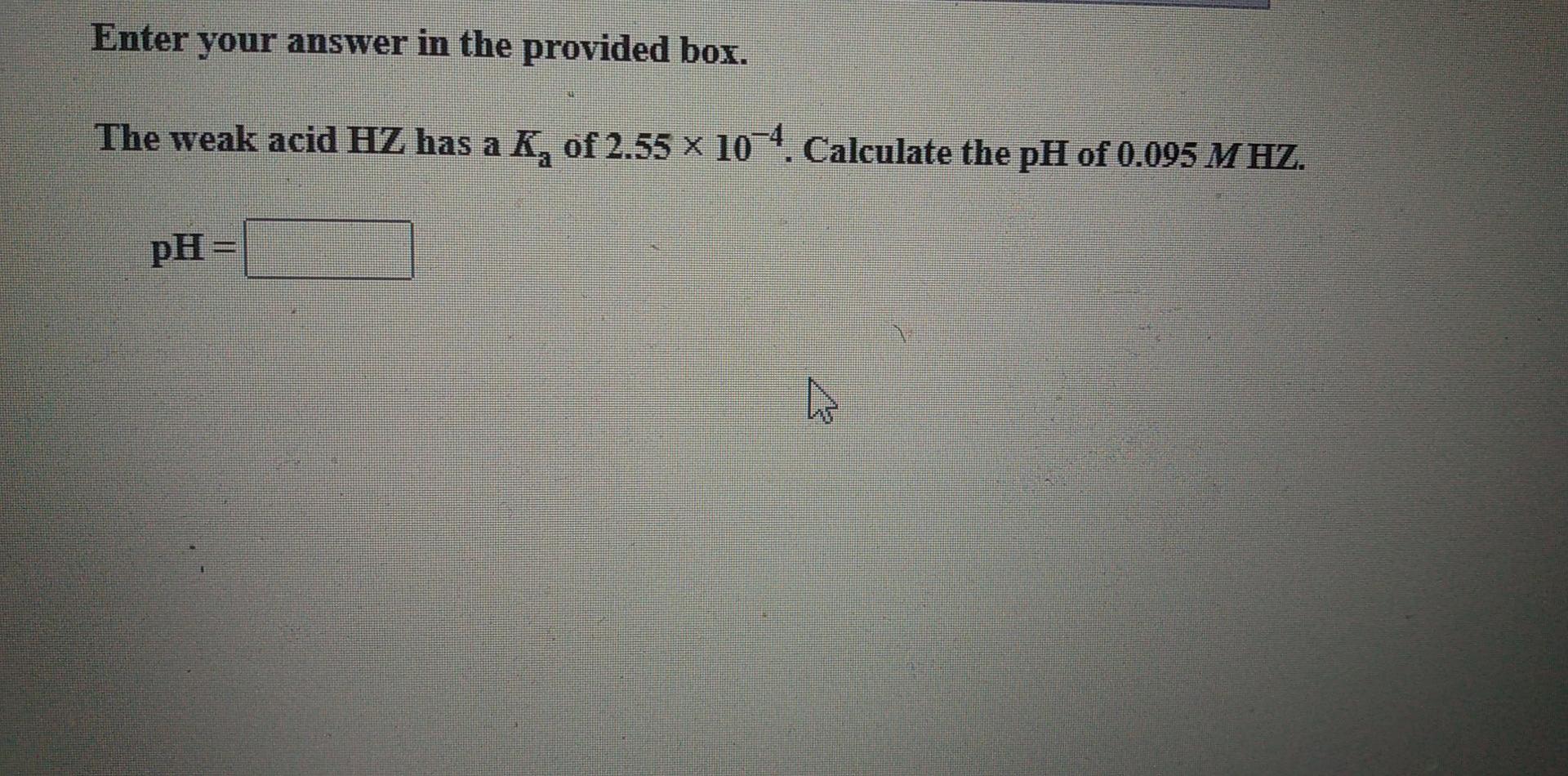
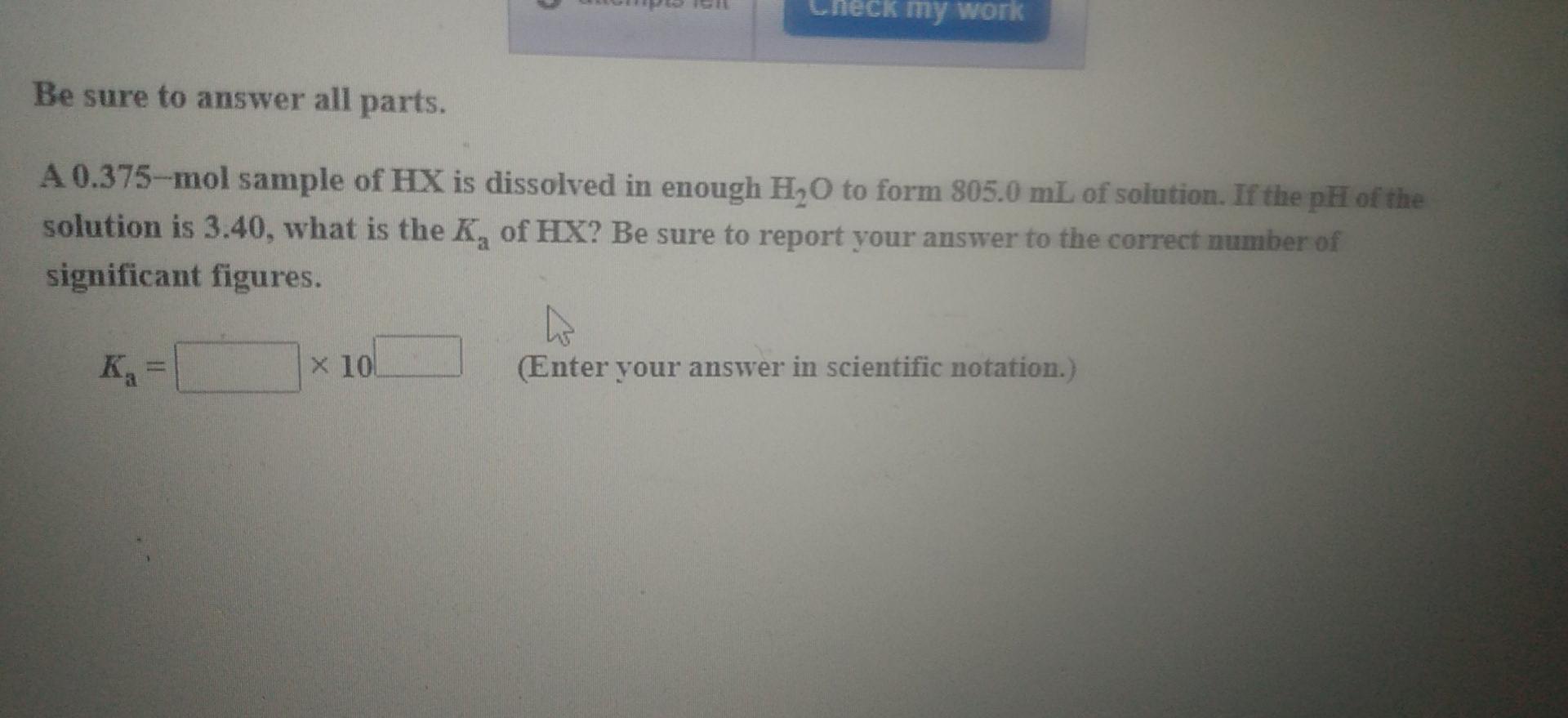
my work Enter your answer in the provided box. The weak acid HZ has a K, of 2.55 x 10- Calculate the pOH of 0.0250 M HZ. pOH = Enter your answer in the provided box. The weak acid HZ has a K, of 2.55 x 10-4. Calculate the pH of 0.095 MHZ. pH = Ck my work Be sure to answer all parts. A 0.375--mol sample of HX is dissolved in enough H,O to form 805.0 mL of solution. If the pH of the solution is 3.40, what is the K, of HX? Be sure to report your answer to the correct number of significant figures. Kg = x 10 (Enter your answer in scientific notation.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


