Question: myNSU x | A Content / Fall 2024 - Chemist x A ALEKS - Kayla Wilkerson - Lee X A Document x : (2676) Women's
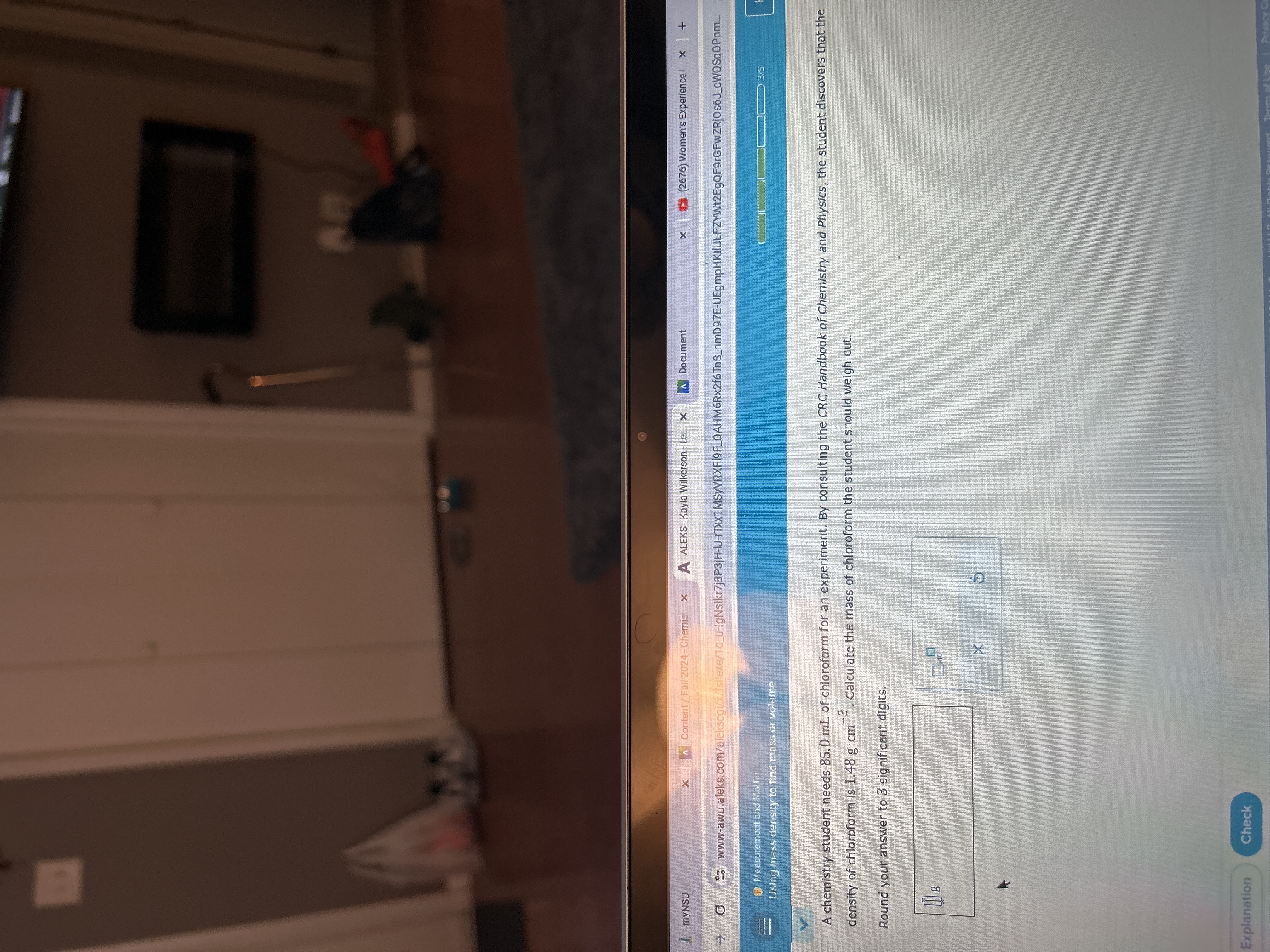
myNSU x | A Content / Fall 2024 - Chemist x A ALEKS - Kayla Wilkerson - Lee X A Document x : (2676) Women's Experience ! x + G So www-awu.aleks.com/alekscgi/x/Islexe/10_u-IgNslkr7j8P3jH-IJ-rTxx1MSyVRXFIOF_OAHM6Rx2f6TnS_nmD97E-UEgmpHKIIULFZYWt2EgQF9rGFwZRjOs6J_cWQSq0Pnm. Measurement and Matter Using mass density to find mass or volume 3/5 V A chemistry student needs 85.0 ml of chloroform for an experiment. By consulting the CRC Handbook of Chemistry and Physics, the student discovers that the density of chloroform is 1.48 g.cm ". Calculate the mass of chloroform the student should weigh out. Round your answer to 3 significant digits. X Explanation Check
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


