Question: N 2 O 4 ( g ) decomposes to NO 2 ( g ) according to the following equation: Pure N 2 O 4 (
N2O4(g) decomposes to NO2(g) according to the following equation:
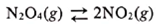
Pure N2O4(g) was placed in a closed flask at 127C at a pressure of 4.38 102 atm. After the system reached equilibrium, the total pressure was found to be 7.43 102 atm. Calculate the value of Kp for this reaction.
N2O4(g)2NO2(g)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


