Question: NA Review | Constants 1 Periodic Table Part A The rate constant for a certain reaction is ke = 8.30x10-35-1. If the initial reactant concentration
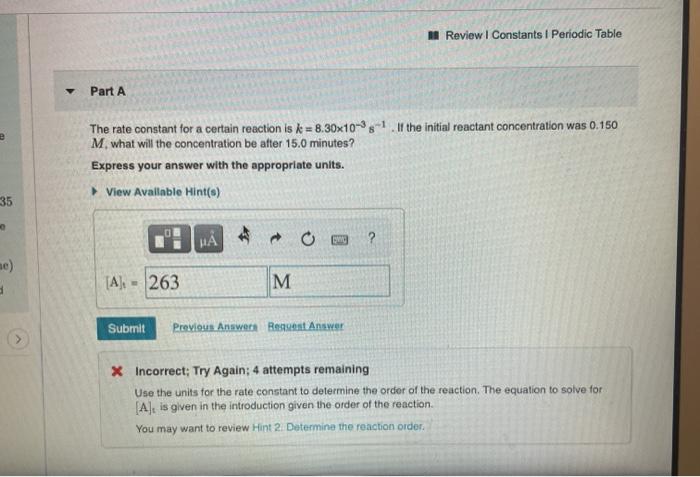
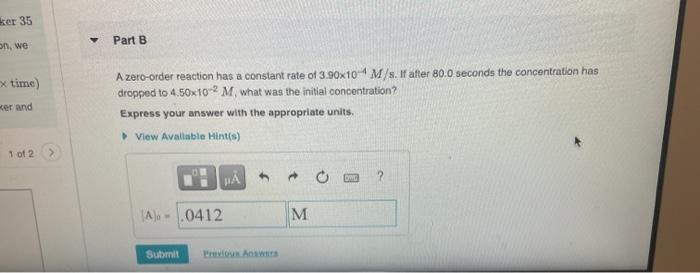
NA Review | Constants 1 Periodic Table Part A The rate constant for a certain reaction is ke = 8.30x10-35-1. If the initial reactant concentration was 0.150 M. what will the concentration be after 15.0 minutes? Express your answer with the appropriate units. View Available Hint() 35 ? , me) [A] 263 M M Submit Previous Answer Regent Answer > X Incorrect; Try Again: 4 attempts remaining Use the units for the rate constant to determine the order of the reaction. The equation to solve for [A], is given in the introduction given the order of the reaction. You may want to review Hint 2. Determine the reaction order ker 35 Part B on, we x time) wer and A zero-order reaction has a constant rate of 3.90x10 4 M/s. If after 80.0 seconds the concentration has dropped to 4.50x10-2M what was the initial concentration? Express your answer with the appropriate units. View Available Hints) 1 of 2 H . ? A) - .0412 M M Submit
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


