Question: Name: Chapter 12 Kinetics Homework 1) (2 pts) Given the balanced equation: 2A1Bry(aq) + 3K_SOs(aq) 6KBr(aq) + Al2(SO4)(aq) and the data below, calculate the average

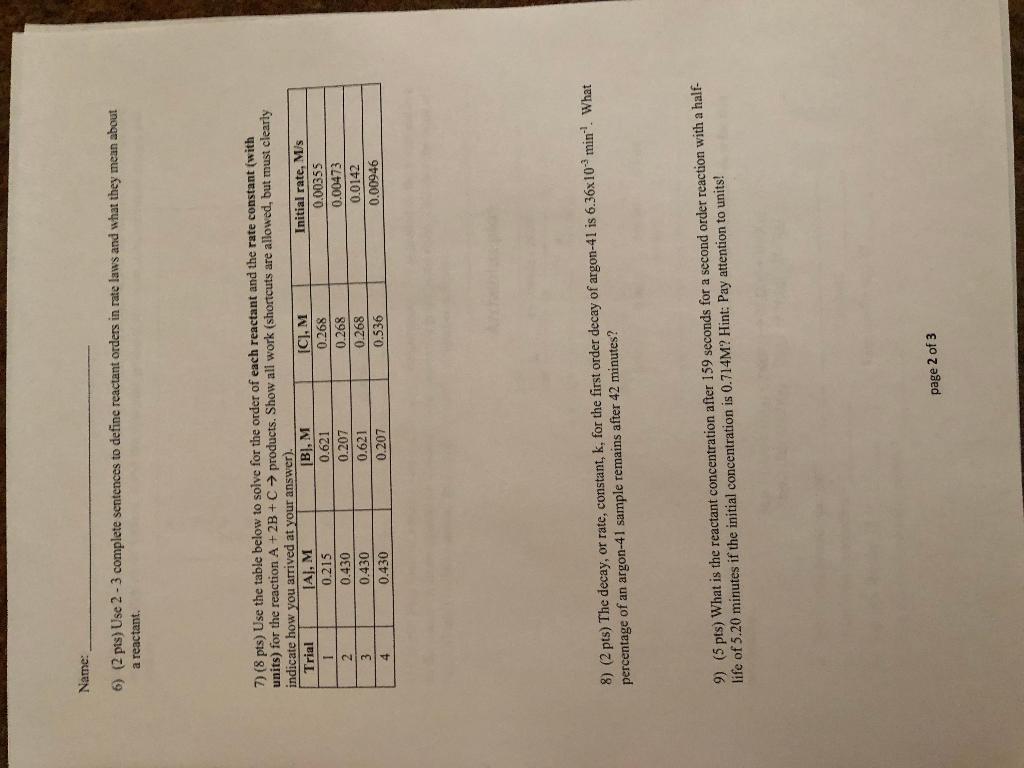

Name: Chapter 12 Kinetics Homework 1) (2 pts) Given the balanced equation: 2A1Bry(aq) + 3K_SOs(aq) 6KBr(aq) + Al2(SO4)(aq) and the data below, calculate the average rate of potassium sulfate (K SO) between 20.0 and 40.0 seconds. Time, s 0.0 10.0 20.0 30.0 40.0 50.0 1K S04), M 0.85 0.76 0.66 0.63 0.62 0.70 2) (2 pts) Use the balanced equation 2A1Br(aq) +3K SO.(aq) 6K Br(aq) + Al(SO4)3(aq) to calculate the rate of reaction if the rate of KBr in a given time period is 0.0248 M/s. 3) (3 pts) Use the balanced equation 2A1Br3(aq) +3K SOH(aq) 6KBr(aq) + Al2(SO4)(aq) to rank the substances in order of slowest to fastest rate of change in a given time interval. Fastest Slowest 4) (6 pts) For the reaction 2X + Y 32, which statement(s) below is/are true? Circle all that apply. a. X disappears twice as fast as Y b. X and Z react at the same rate. c. If the rate of reaction is 0.26 M/s, then the rate of Z in the same time interval is 0.78 M/s. d. Y will react at 1/3 the rate of Z in a given time interval. The rate of reaction will be constant over time. f. X will react at 2/3 the rate of Z. e 5) (4 pts) List four changes that generally increase the rate of reaction, and BRIEFLY state why for each one. page 1 of 3 Name: 6) (2 pts) Use 2 - 3 complete sentences to define reactant orders in rate laws and what they mean about a reactant. 7) (8 pts) Use the table below to solve for the order of each reactant and the rate constant (with units) for the reaction A + 2B + C products. Show all work (shortcuts are allowed, but must clearly indicate how you arrived at your answer). Trial AJ, M [B], M (C), M Initial rate, Mis 1 0.215 0.621 0.268 0.00355 2 0.430 0.207 0.268 0.00473 3 0.430 0.621 0.0142 4 0.430 0.207 0.536 0.00946 0.268 8) (2 pts) The decay, or rate, constant, k, for the first order decay of argon-41 is 6.36x10minWhat percentage of an argon-41 sample remains after 42 minutes? 9) (5 pts) What is the reactant concentration after 159 seconds for a second order reaction with a half- life of 5.20 minutes if the initial concentration is 0.714M? Hint: Pay attention to units! page 2 of 3 Name: 10) (5 pts) Draw a reaction profile diagram for a reaction that has an activation energy of +35 kJ/mol and an enthalpy of -15 kJ/mol. Label the: reactants, products, transition state, activation energy, and enthalpy. 70 60 50 Potential Energy (l) 30 20 10 0 reaction progress 11) (5 pts) Four trials of a reaction run at varying temperatures. 1/T is plotted on the x-axis and Ink on the y-axis. A linear equation is generated as shown on the graph to the right. Calculate the value of E. (in kJ/mol) for this reaction. Report your answer to 3 significant figures. Arrhenius plot -2.00 y = -7285.3x + 19.023 -4.00 Ink -6.00 -8.00 -10.00 0.003 0.0032 0.0034 0.0036 1/T (K) 12) (6 pts) A proposed two-step mechanism is shown below. Answer the questions below for this mechanism. Step 1 (slow): H2(g) + 2NO(g) N2O(g) + H2O(g) Step 2 (fast): N2O(g) + H2(g) N2(g) + H2O(g) a. What is the overall equation? b. What is the intermediate? c. Rate law for step 1? d. Rate law for the overall reaction? Catalyst? Rate law for step 2? page 3 of 3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


