Question: Name Section: 4. (Take home assignment). A set of solution densities as a function of weight/volume % sugar is given below. Note that weight/volume %
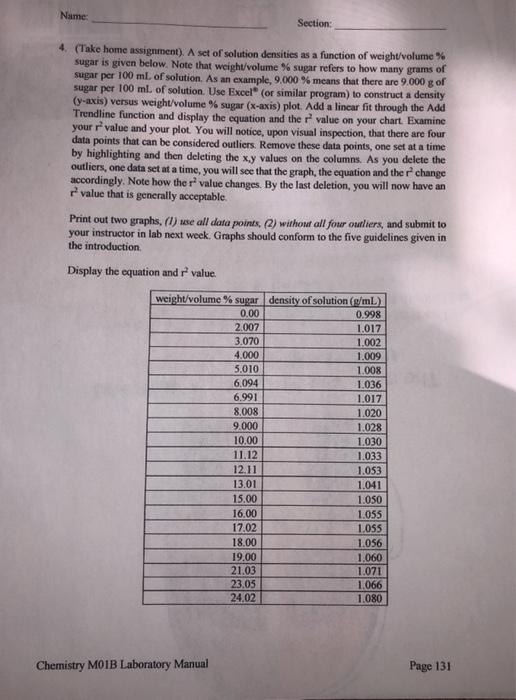
Name Section: 4. (Take home assignment). A set of solution densities as a function of weight/volume % sugar is given below. Note that weight/volume % sugar refers to how many grams of sugar per 100 ml of solution. As an example, 9.000 % means that there are 9.000 g of sugar per 100 ml of solution. Use Excel (or similar program) to construct a density (y-axis) versus weight/volume % sugar (x-axis) plot. Add a linear fit through the Add Trendline function and display the equation and the value on your chart. Examine your value and your plot. You will notice, upon visual inspection, that there are four data points that can be considered outliers. Remove these data points, one set at a time by highlighting and then deleting the xy values on the columns. As you delete the outliers, one data set at a time, you will see that the graph, the equation and the change accordingly. Note how the r value changes. By the last deletion, you will now have an value that is generally acceptable. Print out two graphs, (l) use all data points, (2) without all four outliers, and submit to your instructor in lab next week. Graphs should conform to the five guidelines given in the introduction Display the equation and value. weight/volume % sugar density of solution (o/mL) 0.00 0.998 2.007 1.017 3.070 1.002 4.000 1.009 5.010 1.008 6,094 1.036 6.991 1.017 8,008 1.020 9.000 1.028 10.00 1.030 11.12 1.033 12.11 1.053 13.01 1.041 15.00 1.050 16.00 1.055 17.02 1.055 18.00 1.056 19.00 1.060 21.03 1.071 23.05 1.066 24.02 1.080 Chemistry MOIB Laboratory Manual Page 131
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


