Question: Name: Section: Experiment #3 The Atomic Spectrum of Hydrogen Objective You are going to be calculating the Energy of an electron located at the first
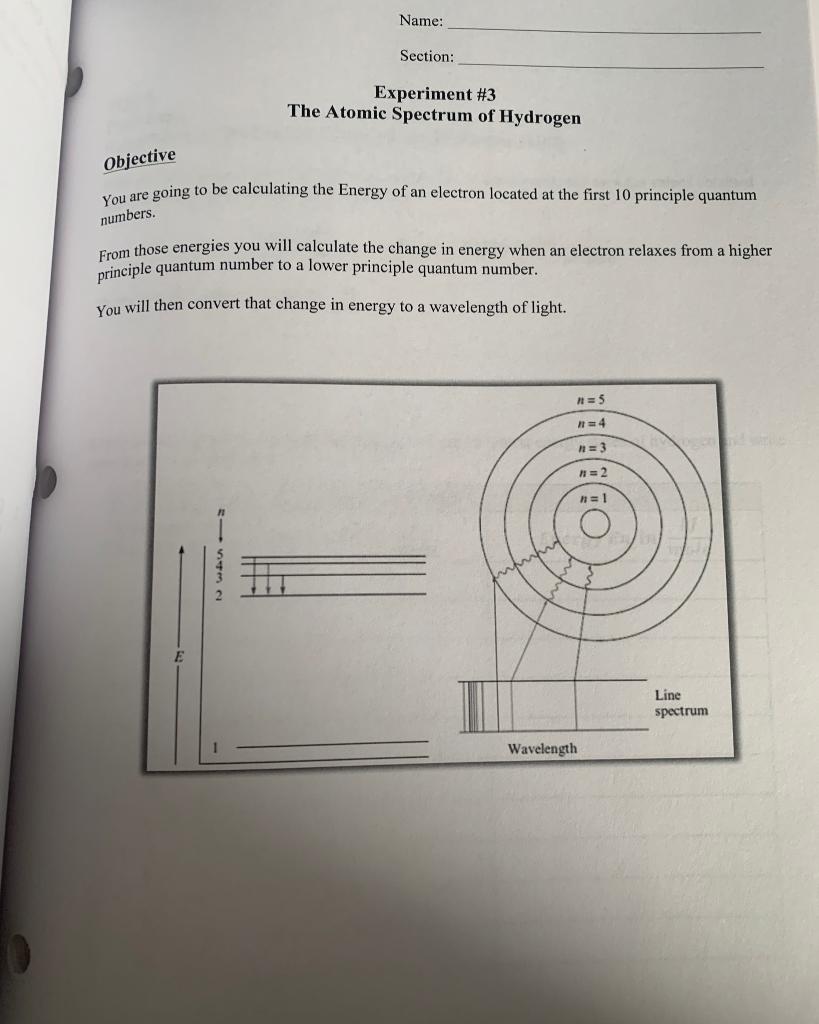
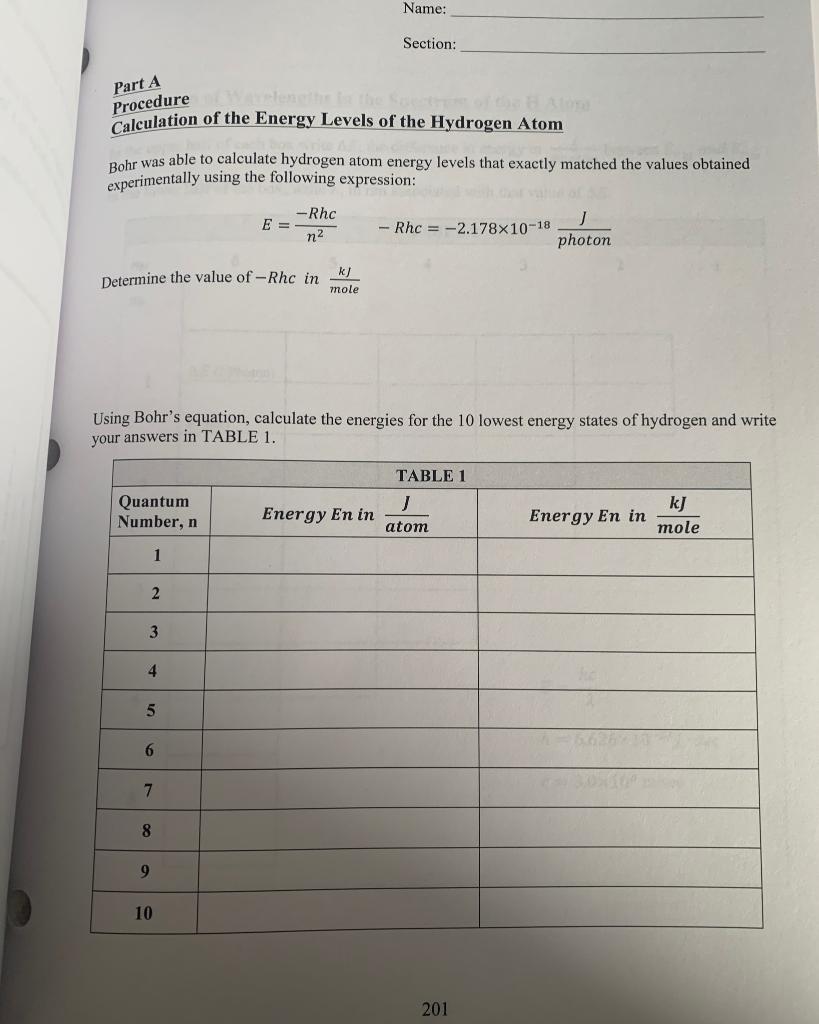
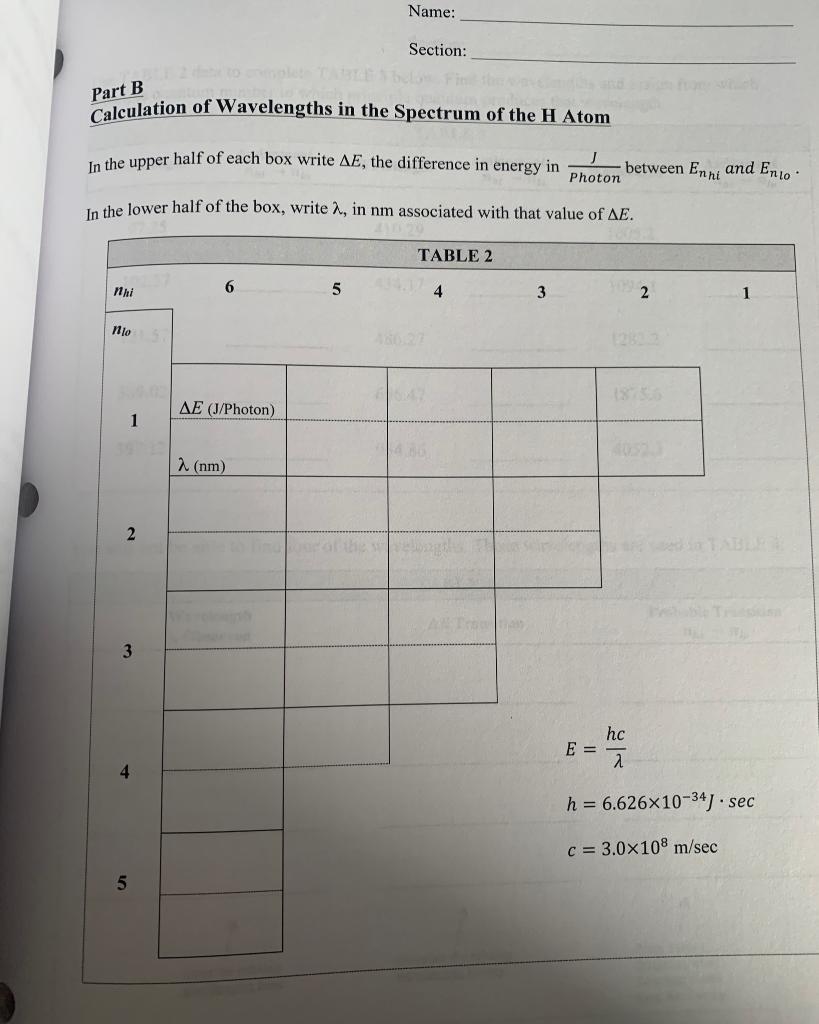
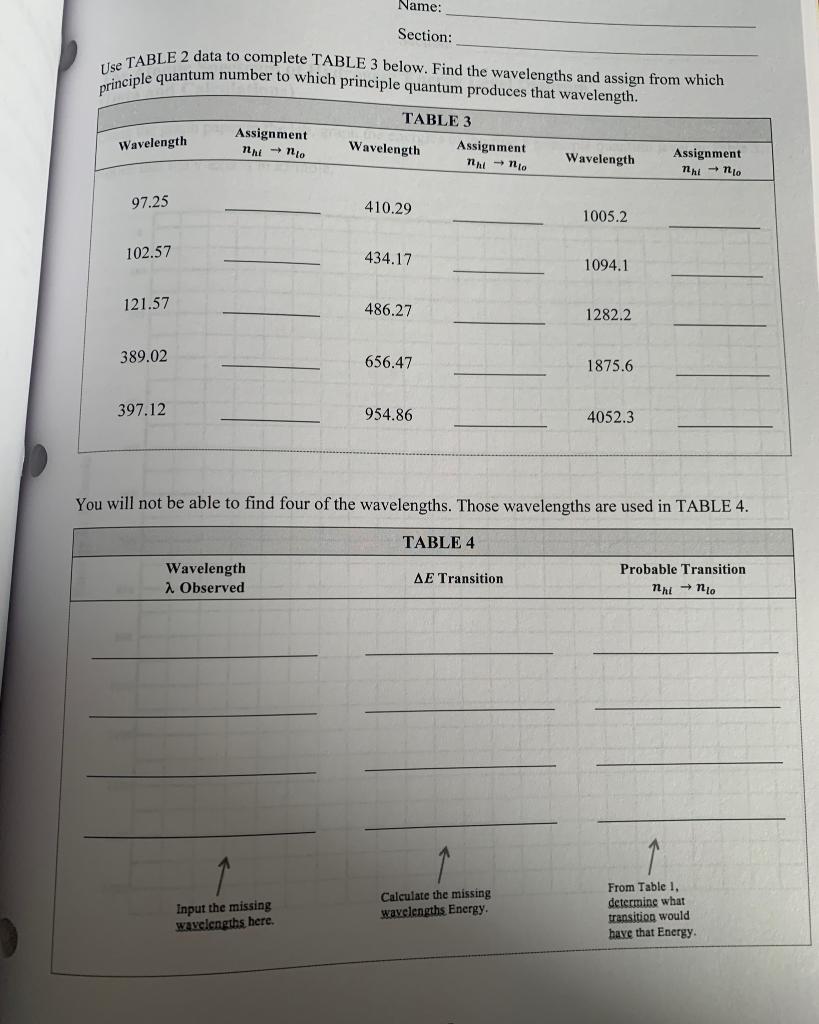
Name: Section: Experiment #3 The Atomic Spectrum of Hydrogen Objective You are going to be calculating the Energy of an electron located at the first 10 principle quantum numbers. From those energies you will calculate the change in energy when an electron relaxes from a higher principle quantum number to a lower principle quantum number. You will then convert that change in energy to a wavelength of light. n = 5 = 4 n=3 Offio H=2 n=1 3432 E Line spectrum Wavelength Name: Section: Part A Procedure et Calculation of the Energy Levels of the Hydrogen Atom Bohr was able to calculate hydrogen atom energy levels that exactly matched the values obtained experimentally using the following expression: -Rhc E = -Rhc = -2.178x10-18 photon n2 Determine the value of -Rhc in k] mole Using Bohr's equation, calculate the energies for the 10 lowest energy states of hydrogen and write your answers in TABLE 1. Quantum Number, n TABLE 1 J atom Energy En in kJ Energy En in mole 1 2 3 4 5 6 7 8 9 10 201 Name: Section: Part B Calculation of Wavelengths in the Spectrum of the H Atom In the upper half of each box write AE, the difference in energy in between Enni and Ento Photon In the lower half of the box, write a., in nm associated with that value of AE. TABLE 2 nhi 6 5 4 3 2 1 no AE (J/Photon) 1 2 (nm) 2 3 hc E = 2 h = 6.626x10-34) sec c= 3.0x108 m/sec 5 Name: Section: Use TABLE 2 data to complete TABLE 3 below. Find the wavelengths and assign from which principle quantum number to which principle quantum produces that wavelength. TABLE 3 Assignment Wavelength Assignment Assignment nni - no Wavelength nento nh - no Wavelength 97.25 410.29 1005.2 102.57 434.17 1094.1 121.57 486.27 1282.2 389.02 656.47 1875.6 397.12 954.86 4052.3 You will not be able to find four of the wavelengths. Those wavelengths are used in TABLE 4. TABLE4 Wavelength 1 Observed AE Transition Probable Transition nhi no Input the missing wavelengths here. Calculate the missing wavelengths Energy From Table 1, determine what transition would have that Energy Section: The Atomic Spectrum of Hydrogen: Energy Level Diagram (Data and Calculations) Using the graph paper in the lab, graph the energies of the first 5 principle quantum level in Table 3. Notice that the y-axis is in kJ/mole. 0 100 200 300 400 500 600 Energy in kJ/mole 700 800 900 1000 1100 1200 1300 1400 Name: Section: Experiment #3 The Atomic Spectrum of Hydrogen Objective You are going to be calculating the Energy of an electron located at the first 10 principle quantum numbers. From those energies you will calculate the change in energy when an electron relaxes from a higher principle quantum number to a lower principle quantum number. You will then convert that change in energy to a wavelength of light. n = 5 = 4 n=3 Offio H=2 n=1 3432 E Line spectrum Wavelength Name: Section: Part A Procedure et Calculation of the Energy Levels of the Hydrogen Atom Bohr was able to calculate hydrogen atom energy levels that exactly matched the values obtained experimentally using the following expression: -Rhc E = -Rhc = -2.178x10-18 photon n2 Determine the value of -Rhc in k] mole Using Bohr's equation, calculate the energies for the 10 lowest energy states of hydrogen and write your answers in TABLE 1. Quantum Number, n TABLE 1 J atom Energy En in kJ Energy En in mole 1 2 3 4 5 6 7 8 9 10 201 Name: Section: Part B Calculation of Wavelengths in the Spectrum of the H Atom In the upper half of each box write AE, the difference in energy in between Enni and Ento Photon In the lower half of the box, write a., in nm associated with that value of AE. TABLE 2 nhi 6 5 4 3 2 1 no AE (J/Photon) 1 2 (nm) 2 3 hc E = 2 h = 6.626x10-34) sec c= 3.0x108 m/sec 5 Name: Section: Use TABLE 2 data to complete TABLE 3 below. Find the wavelengths and assign from which principle quantum number to which principle quantum produces that wavelength. TABLE 3 Assignment Wavelength Assignment Assignment nni - no Wavelength nento nh - no Wavelength 97.25 410.29 1005.2 102.57 434.17 1094.1 121.57 486.27 1282.2 389.02 656.47 1875.6 397.12 954.86 4052.3 You will not be able to find four of the wavelengths. Those wavelengths are used in TABLE 4. TABLE4 Wavelength 1 Observed AE Transition Probable Transition nhi no Input the missing wavelengths here. Calculate the missing wavelengths Energy From Table 1, determine what transition would have that Energy Section: The Atomic Spectrum of Hydrogen: Energy Level Diagram (Data and Calculations) Using the graph paper in the lab, graph the energies of the first 5 principle quantum level in Table 3. Notice that the y-axis is in kJ/mole. 0 100 200 300 400 500 600 Energy in kJ/mole 700 800 900 1000 1100 1200 1300 1400
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


