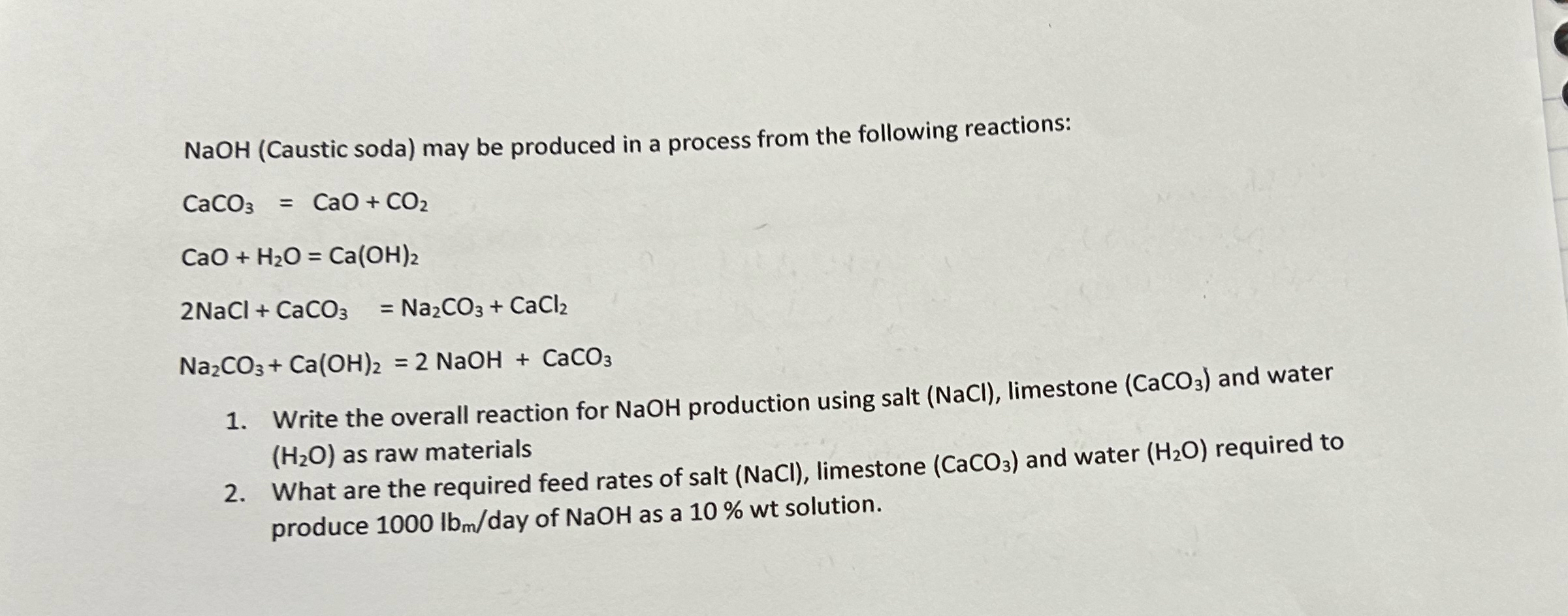
Question: NaOH ( Caustic soda ) may be produced in a process from the following reactions: C a C O 3 = CaO + C O
NaOH Caustic soda may be produced in a process from the following reactions:
CaO
CaO
NaCl
NaOH
Write the overall reaction for NaOH production using salt limestone and water as raw materials
What are the required feed rates of salt limestone and water required to produce day of NaOH as a wt solution.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


