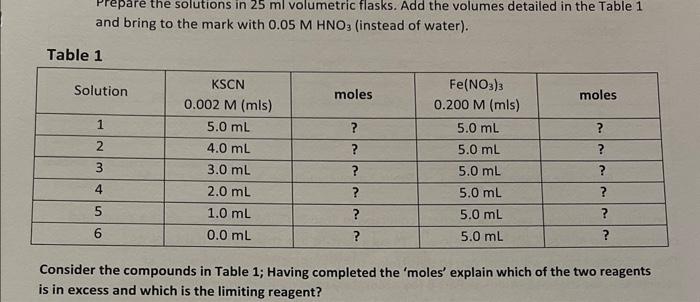
Question: - NB: Complete the calculations (Table 1) before entering the laboratory (show calculations to your Demonstrator). - While both reagents KSCN and Fe(NO3)3 in Table

![in Table 1 will contribute to the formation of the complex [Fe(SCN)]2+](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f90e2c121e4_12366f90e2baebd0.jpg)
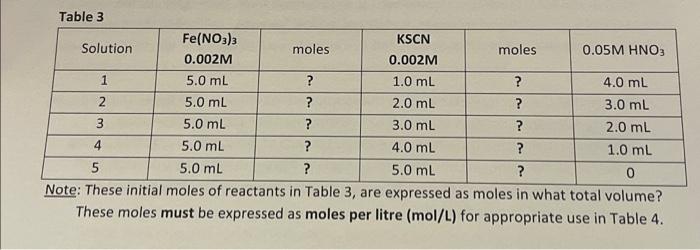
- NB: Complete the calculations (Table 1) before entering the laboratory (show calculations to your Demonstrator). - While both reagents KSCN and Fe(NO3)3 in Table 1 will contribute to the formation of the complex [Fe(SCN)]2+ in Table 2, determine which of these 2 reagents (column 2 ? or column 3?) from Table 1, will determine the actual quantity of [Fe(SCN)]2+ formed in Table 2? Prepare the solutions in 25ml volumetric flasks. Add the volumes detailed in the Table 1 and bring to the mark with 0.05MHNO3 (instead of water). Table 1 Consider the compounds in Table 1; Having completed the 'moles' explain which of the two reagents is in excess and which is the limiting reagent? Table 2 Note: The square brackets, [ ], Table 2 above, indicate 'mol/L' (molarity; M). Tahle 2 Note: These initial moles of reactants in Table 3, are expressed as moles in what total volume? These moles must be expressed as moles per litre (mol/L) for appropriate use in Table 4
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


