Question: need explanation for question 4 a and b as well. 4. Acidity and Basioity A. For the following reaction place an arrow from the side
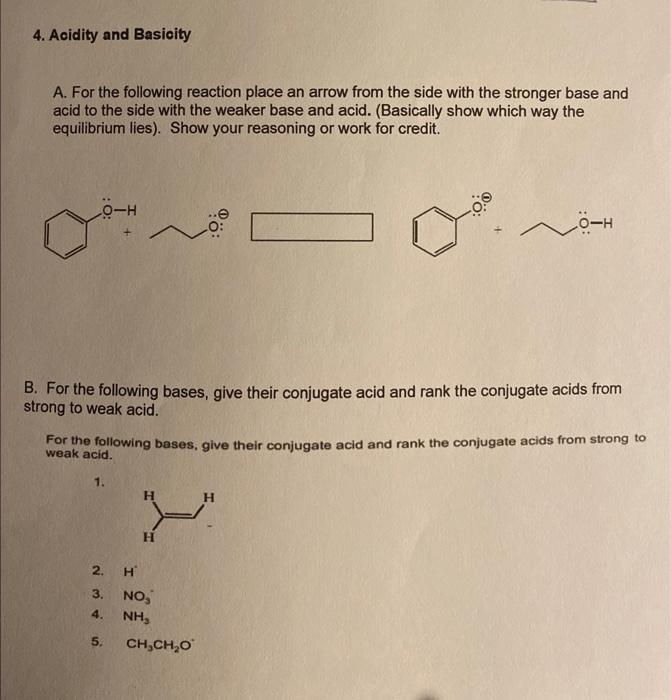
4. Acidity and Basioity A. For the following reaction place an arrow from the side with the stronger base and acid to the side with the weaker base and acid. (Basically show which way the equilibrium lies). Show your reasoning or work for credit. B. For the following bases, give their conjugate acid and rank the conjugate acids from strong to weak acid. For the following bases, give their conjugate acid and rank the conjugate acids from strong to weak acid. 1. 2. H 3. NO3 4. NH3 5. CH3CH2O
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


