Question: Need full work pls - thermodynamics problem An important mechanism for controlling the temperature of the human body is perspiration and subsequent evaporation. Convert the
Need full work pls - thermodynamics problem
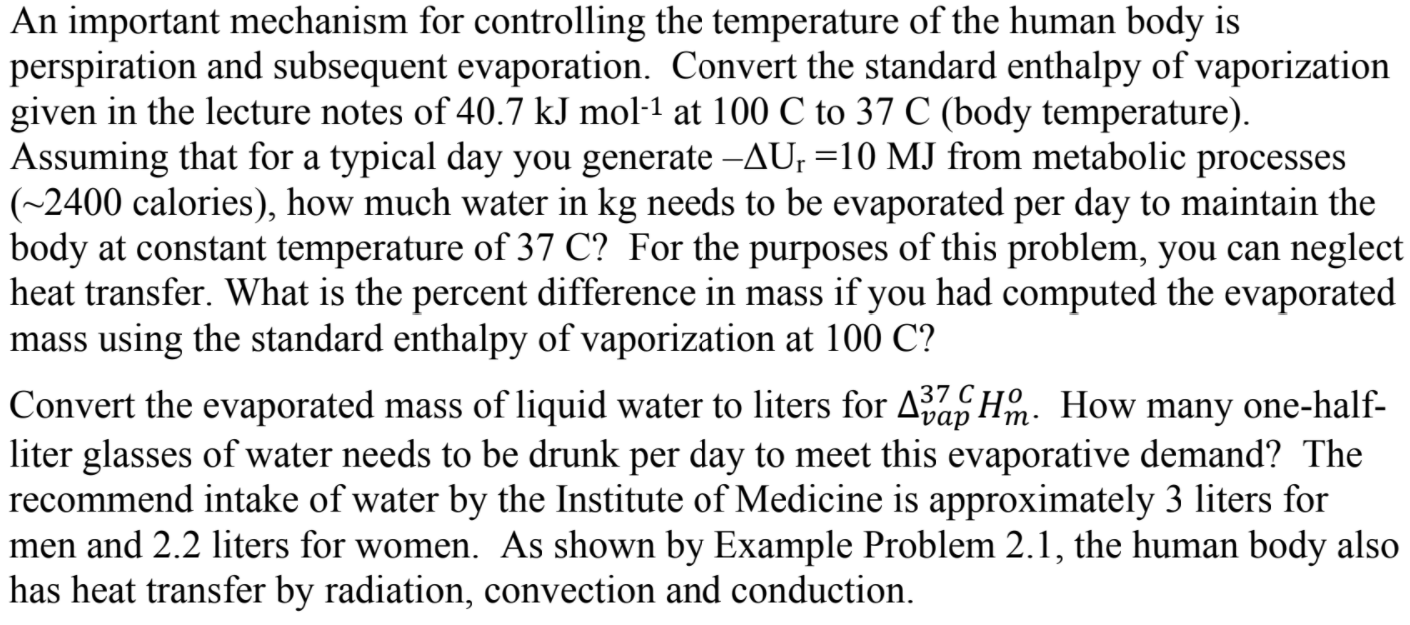
An important mechanism for controlling the temperature of the human body is perspiration and subsequent evaporation. Convert the standard enthalpy of vaporization given in the lecture notes of 40.7 kJ mol-1 at 100 C to 37 C (body temperature). Assuming that for a typical day you generate AU, =10 MJ from metabolic processes (~2400 calories), how much water in kg needs to be evaporated per day to maintain the body at constant temperature of 37 C? For the purposes of this problem, you can neglect heat transfer. What is the percent difference in mass if you had computed the evaporated mass using the standard enthalpy of vaporization at 100 C? Convert the evaporated mass of liquid water to liters for Ajap Hm. How many one-half- liter glasses of water needs to be drunk per day to meet this evaporative demand? The recommend intake of water by the Institute of Medicine is approximately 3 liters for men and 2.2 liters for women. As shown by Example Problem 2.1, the human body also has heat transfer by radiation, convection and conduction. 37 C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


