Question: need help 1-4 Data Table It: Sample Weights Item Weight of Dish/ (grams) Weight of Dish Plus Aspirin Sample (grams) Net Weight of Aspirin Sample
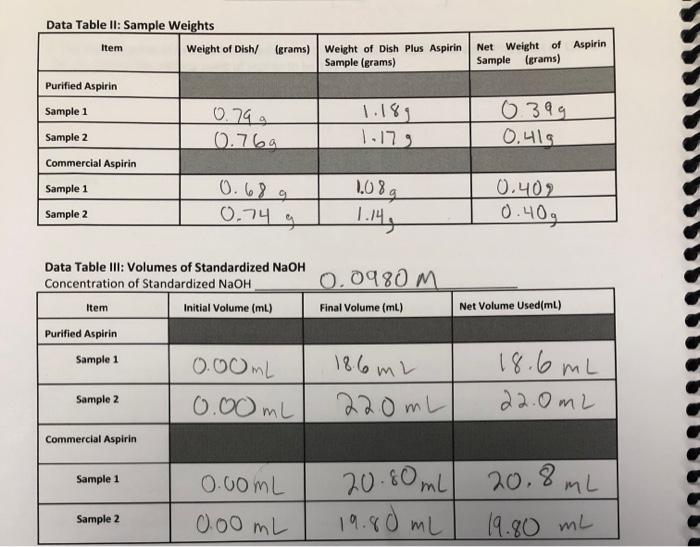

Data Table It: Sample Weights Item Weight of Dish/ (grams) Weight of Dish Plus Aspirin Sample (grams) Net Weight of Aspirin Sample (grams) 0.79 g 0.7ba 1.18, 1.179 0399 0.419 Purified Aspirin Sample 1 Sample 2 Commercial Aspirin Sample 1 Sample 2 1.08 0.689 0.74 g 1.146 0.409 0.40g 0.0980 M Data Table III: Volumes of Standardized NaOH Concentration of Standardized NaOH Item Initial Volume (mL) Purified Aspirin Sample 1 Final Volume (ml) Net Volume Used(ml) 18.66 m 0.00mL 0.00 mL 18.6 mL 22.0mL Sample 2 220mL Commercial Aspirin Sample 1 0.00ML 20.80mL 20.8mL Sample 2 0.00 mL 19.80 mL 19.80 mL Concentration of NaOH (from jug in tab)_ 0.0980 m From your titration data, calculate the percent purity for each of the three purified and each of the three commercial samples. Include all units in these calculations, Purified Aspirin Sample 1. 1. 2. Purified Aspirin Sample 2. Average % purity of Aspirin = 3. Commercial Aspirin Sample 1. hoogmo 4. Commercial Aspirin Sample 2. Average % purity of Commercial aspirin =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


