Question: need help #3 3. (a) How must the two reactions above be combined to provide the equilibrium N2048) 2NO2(g)? Show in detail, the mathematical steps
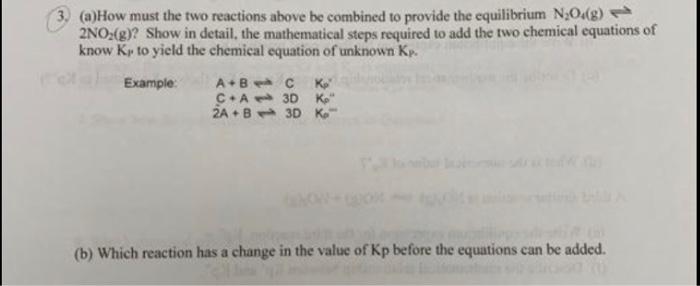
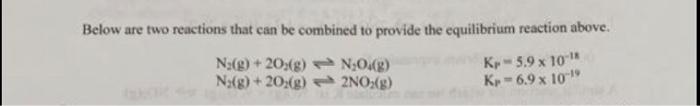
3. (a) How must the two reactions above be combined to provide the equilibrium N2048) 2NO2(g)? Show in detail, the mathematical steps required to add the two chemical equations of know Kp to yield the chemical equation of unknown Kp. Example ABC ko CHA 3D ko" 2A- B3D Ko (b) Which reaction has a change in the value of Kp before the equations can be added. Below are two reactions that can be combined to provide the equilibrium reaction above. N:(g) + 20%(8) N:O4() Nx(g) + 2O2(g) 2NO2(g) K-5.9 x 10" K-6.9 x 10-19
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


