Question: need help Consider the gas plase raction between nitric oxide and bromiae at 273C : H2SeOina+6I(a0+4HsiSe91+2Iias+3H2O The following kinetic data for the initial rate of
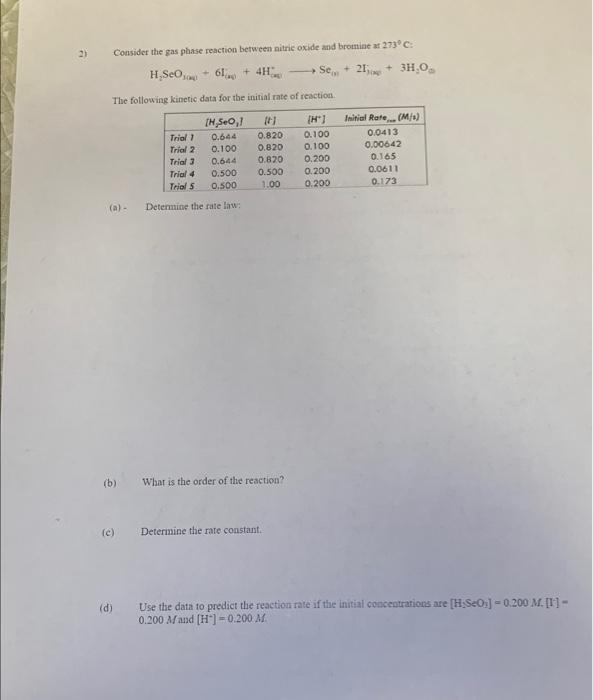
Consider the gas plase raction between nitric oxide and bromiae at 273C : H2SeOina+6I(a0+4HsiSe91+2Iias+3H2O The following kinetic data for the initial rate of reaction. (a) - Determine the rate law: (b) What is the order of the reaction? (c) Determine the rate constant. (d) Use the data to predict the reaction rate if the initial cencentrations are [H2SeOy]=0.200M. [I-] = 0.200M and [H]=0.200M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


