Question: Need help filling out this chart with densities. Pre lab information: PART B: Density of the Solution Measuring Volume with a Volumetric Pipet Obtain about
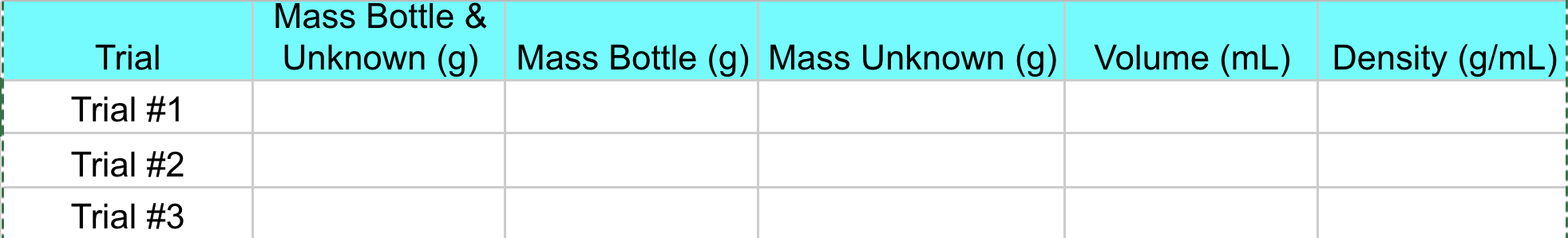
Need help filling out this chart with densities.
Pre lab information:
PART B: Density of the Solution Measuring Volume with a Volumetric Pipet
Obtain about 50 mL of your unknown solution from the instructor to use for the entire experiment.
Weigh a labeled empty weigh bottle with cap.
Pipet 10 mL of the unknown solution into the weigh bottle.
Re-weigh the bottle with solution. Obtain the mass of the solution.
Take the temperature of the solution.
Determine the density of the solution.
Repeat steps 1 4 two more times so you have three trials in all.
As with all glassware, please be sure to clean the bottles and pipets well with d.i. water (rinse three times) before returning them to the Common Use areas.
Also find: Average Std Dev RSD
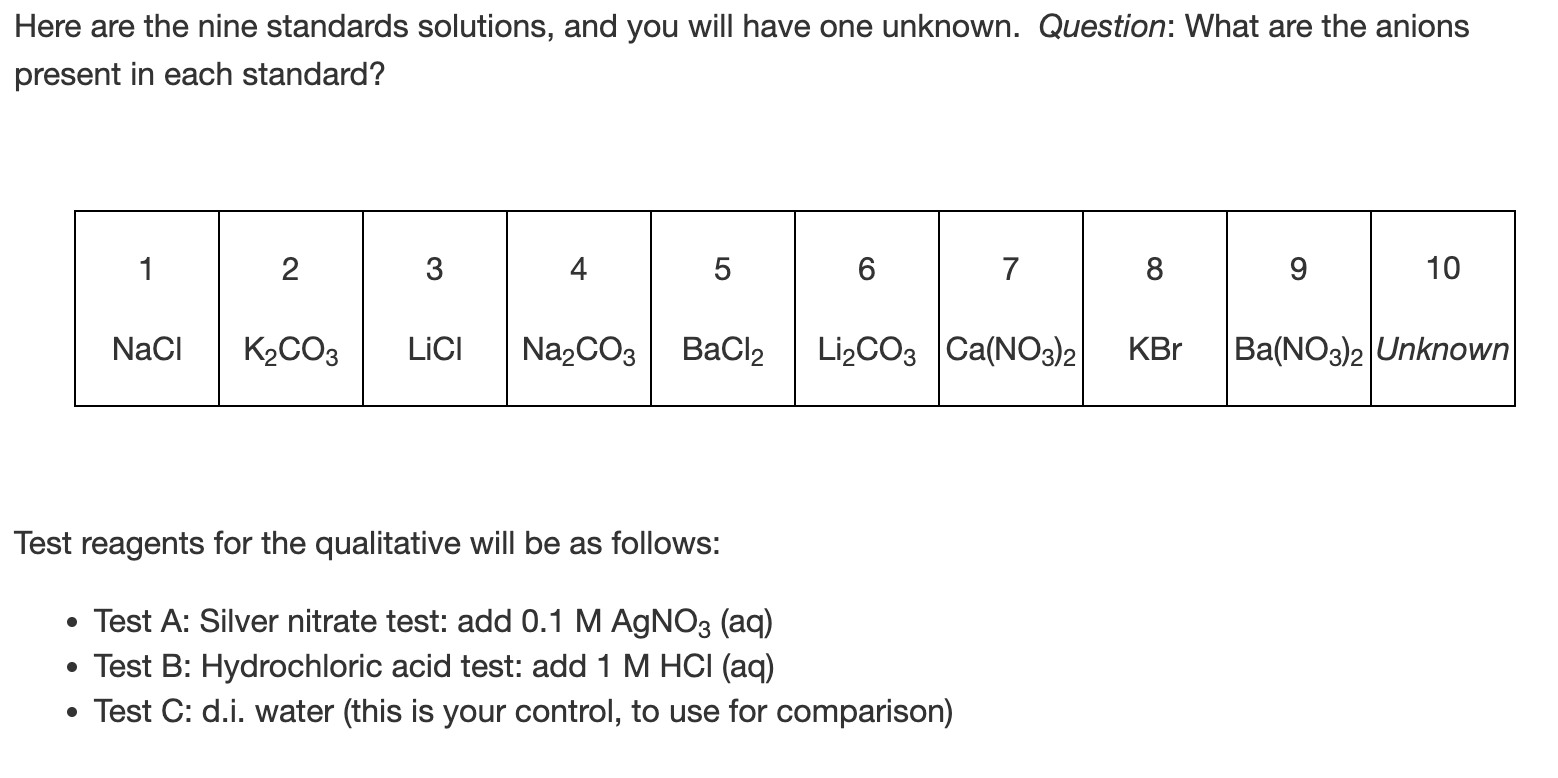
Mass Bottle \& Unknown (g) Mass Bottle (g) Mass Unknown (g) Volume (mL) Density (g/mL) Trial \#1 Trial \#2 Trial \#3 Here are the nine standards solutions, and you will have one unknown. Question: What are the anions present in each standard? Test reagents for the qualitative will be as follows: - Test A: Silver nitrate test: add 0.1MAgNO3 (aq) - Test B: Hydrochloric acid test: add 1MHCl(aq) - Test C: d.i. water (this is your control, to use for comparison) Mass Bottle \& Unknown (g) Mass Bottle (g) Mass Unknown (g) Volume (mL) Density (g/mL) Trial \#1 Trial \#2 Trial \#3 Here are the nine standards solutions, and you will have one unknown. Question: What are the anions present in each standard? Test reagents for the qualitative will be as follows: - Test A: Silver nitrate test: add 0.1MAgNO3 (aq) - Test B: Hydrochloric acid test: add 1MHCl(aq) - Test C: d.i. water (this is your control, to use for comparison)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


