Question: Need help for second question that is marked incorrect, thank you!! A 0.118m aqueous solution of LiCl has an experimentally measured freezing point of 0.415C.
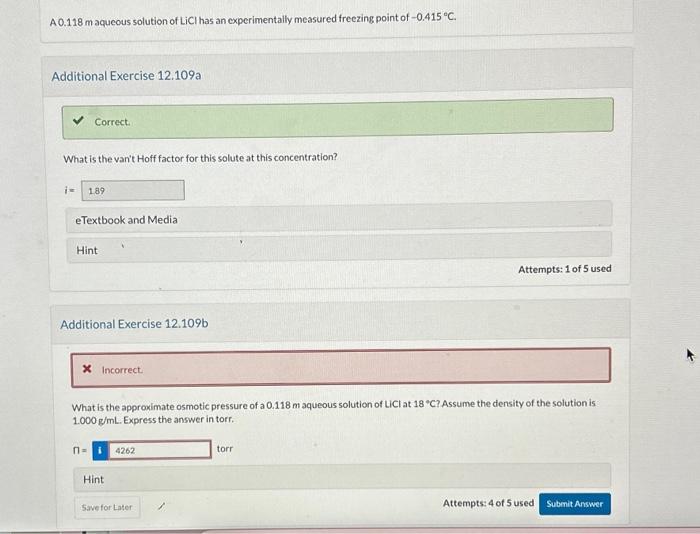
A 0.118m aqueous solution of LiCl has an experimentally measured freezing point of 0.415C. Additional Exercise 12.109 What is the van't Hoff factor for this solute at this concentration? i= GTavthank and Morlis Additional Exercise 12.109b What is the approximate osmotic pressure of a 0.118m aqueous solution of LiCl at 18C ? Assume the density of the solution is 1.000g/mL. Express the answer in torr
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


