Question: need help in all 3 please!!!! Consider the following system, for which H=10.4kJ, at equilibrium at 698K : 2HI(g)?H2(g)+I2(g) The production of H2(g) is favored


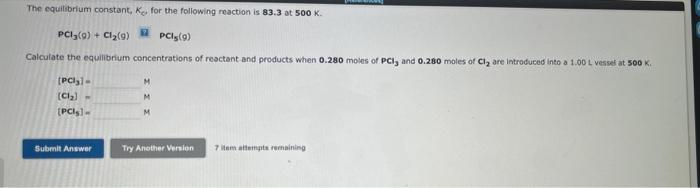
Consider the following system, for which H=10.4kJ, at equilibrium at 698K : 2HI(g)?H2(g)+I2(g) The production of H2(g) is favored by: 1. Decreasing the temperature. 2. Increasing the pressure (by changing the volume). 3. Decreasing the volume. 4. Removing HI. 5. Removing I2. The equllibrium constant, Kc, for the following reaction is 1.20102 at 500K. Calculate the equilibrium concentrations of reactant and products when 0.348 moles of PCl5(9) are introduced into a 1.00L vessel at 500K. [PCl5]=[PCl3]=[Cl2]=MMM 10 item attempte remaining The equilibrium constant, K, for the following reaction is 83.3 at 500K. PCl3(g)+Cl2(g)PCl5(g) Calculate the equilibrium concentrations of reactant and products when 0.280 moles of PCl3 and 0.280molesisCl2 are introduced into a 1.00L vessef at soo K.. [PCl3]=[Cl2]=[PCls]=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


