Question: Need help in finishing the excercise for my java class 4: Formic acid (HCOOH) is a relatively strong weak acid that ants use for defense.
 Need help in finishing the excercise for my java class
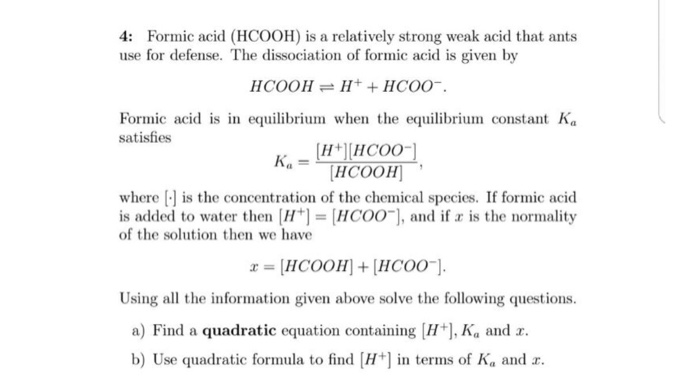
Need help in finishing the excercise for my java class 4: Formic acid (HCOOH) is a relatively strong weak acid that ants use for defense. The dissociation of formic acid is given by Formic acids satisfies equilibrium when the equilibrium constant Ka K.= HCOOH where is the concentration of the chemical species. If formic acid is added to water then [H+] = [HC00-], and if x is the normality of the solution then we have x = [HCOOH] + [HCOO-]. Using all the information given above solve the following questions. a) Find a quadratic equation containing [H], Ka and b) Use quadratic formula to find H+ in terms of Ka and
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


