Question: need help on both 11 and 12 please 11. When I was 9 years old, a paper was published on the thermodynamics of the Krebs


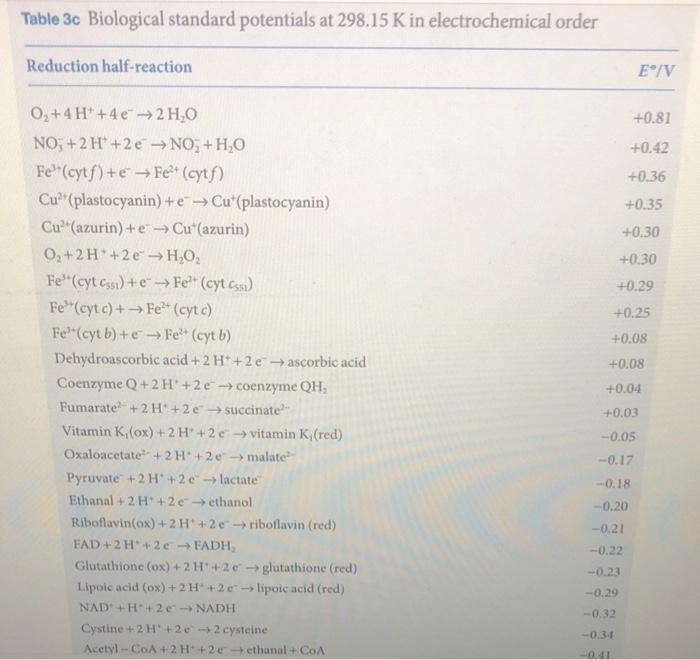
11. When I was 9 years old, a paper was published on the thermodynamics of the Krebs cycle and related compounds. The paper is very rigorous, taking into account many processes under a variety of conditions. In one of the tables, I found rG for succinic acid and fumaric acid. The values are as follows; rG suc =690kJ/mol and rG fum =604.5 kJ/mol. Using these values, can you justify the biological standard potential for the half-reaction in the back of your book (page 572) where fumarate is being reduced to succinate? 12. Couple the half reaction in the above problem to that of FAD/FADH/2 (same A page) and determine the equilibrium constant for the overall reaction. Fe3+(cytb)+eFe2+(cytb) Dehydroascorbic acid +2H++2e ascorbic acid Coenzyme Q+2H++2e coenzyme QH2 Fumarate 2+2H++2e succinate 2 Vitamin K1(ox)+2H++2e vitamin K1 (red) +0.08 +0.08 Oxaloacetate 2+2H++2e malate 2 +0.04 Pyruvate +2H++2e lactate +0.03 Ethanal +2H++2e ethanol 0.05 Riboflavin (ox)+2H++2e riboflavin (red) 0.17 FAD+2H++2eFADH2 0.18 Glutathione (ox) +2H++2e glutathione (red) 0.20 Lipoic acid (ox) +2H++2e lipoic acid (red) 0.21 NAD++H++2eNADH 0.22 Cystine +2H++2e2 cysteine 0.23 Acetyl CoA+2H++2e ethanal +CoA 0.29 0.32 2H2O+2eH2+2OH 0.34 Ferredoxin (ox) +e ferredoxin (red) 0.41 0.42 O2+eO2 0.43 0.4 Table 3c Biological standard potentials at 298.15K in electrochemical order Reduction half-reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


