Question: need help on homework please A chemist is studying the following eqoilibirum, which has the given equilibrum constant at a certain temperature: 2CH4(g)C2H2(g)+3H2(g)KF=5,42104 He filts
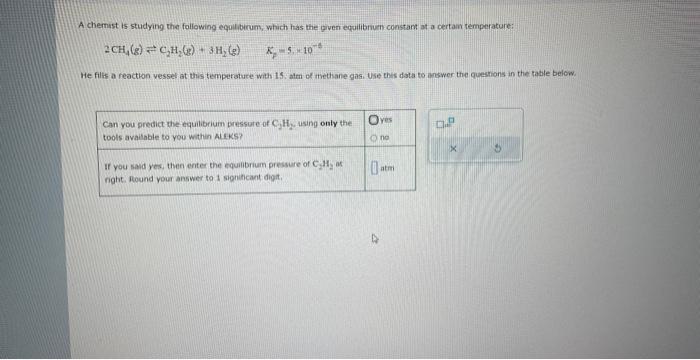
A chemist is studying the following eqoilibirum, which has the given equilibrum constant at a certain temperature: 2CH4(g)C2H2(g)+3H2(g)KF=5,42104 He filts a reaction vessel at this temperature with 15. aten of ithethane gas. Use this data to answer the questions in the table below
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


