Question: need help on the rest asap lmk if lab report is needed! thank you 79 Experiment 6: Acid/Base Titration: Percent Composition of Carbonate/Bicarbonate in an
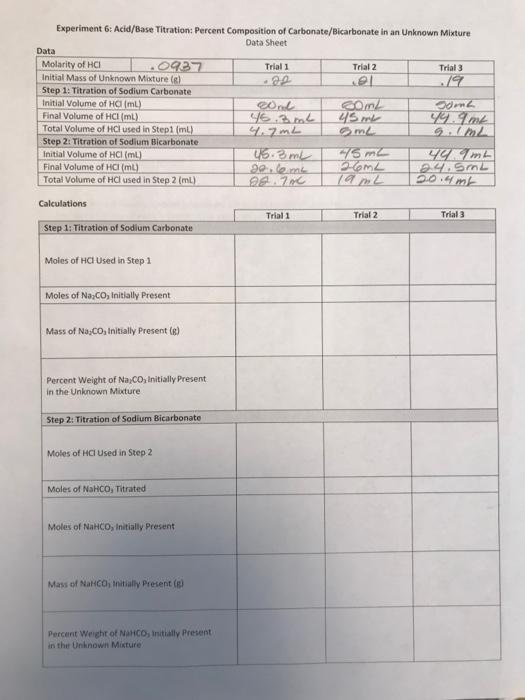

79 Experiment 6: Acid/Base Titration: Percent Composition of Carbonate/Bicarbonate in an unknown Mixture Data Sheet Data Molarity of HCI .0927 Trial 1 Trial 2 Trial 3 Initial Mass of Unknown Mixtures) .22 el Step 1: Titration of Sodium Carbonate Initial Volume of HamL) ond com soma Final Volume of HCI () 45.3 mL 45 m 49.9m Total Volume of HCl used in Step1 mi) 4.7mL 9..2 Step 2: Titration of Sodium Bicarbonate Initial volume of HCl(ml) 45 m2 Ym Final Volume of HCl (ml) gelem 26m2 2.4. Srn2 Total Volume of HCl used in Step 2 (mL) 28.700 Calculations Trial 1 Trial 2 Trial 3 Step 1: Titration of Sodium Carbonate Moles of HCl Used in Step 1 Moles of NaCo Initially Present Mass of No Co.Initially Present (e) Percent Weight of Na,Co, Initially Present in the Unknown Mixture Step 2: Titration of Sodium Bicarbonate Moles of HCI Used in Step 2 Moles of NaHCO, Titrated Moles of NaHCO, initially Present Mass of Narco, initially Present (el Percent Weight of NaHCO, initially Present in the Unknown Mixture Step 3: Determine the Mass of Neutral Component Mass of Neutral component to Percent Weight of Neutral Component initially Present in the Unknown Mature Average Percent Weight for All Determinations Na,CO3 NaHCO3 Neutral Component Results and Discussion of Results State the results for each part of the experiment. Which aspects of the reperiment made it difficult for you to accurately determine the percent weight of each component in the unknown mixture? if you elected to exclude the results from any individual trial of the experiment from the final results and/or calculations, please explain your rationale
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


