Question: need help on these 2 questions fast please. and make sure it's right for a thumbs up please. MAKE SURE YOU HAVE THE ANSWER AND
need help on these 2 questions fast please. and make sure it's right for a thumbs up please. MAKE SURE YOU HAVE THE ANSWER AND THE UNITS FOR THE QUESTION WHEN IT'S ASKED.
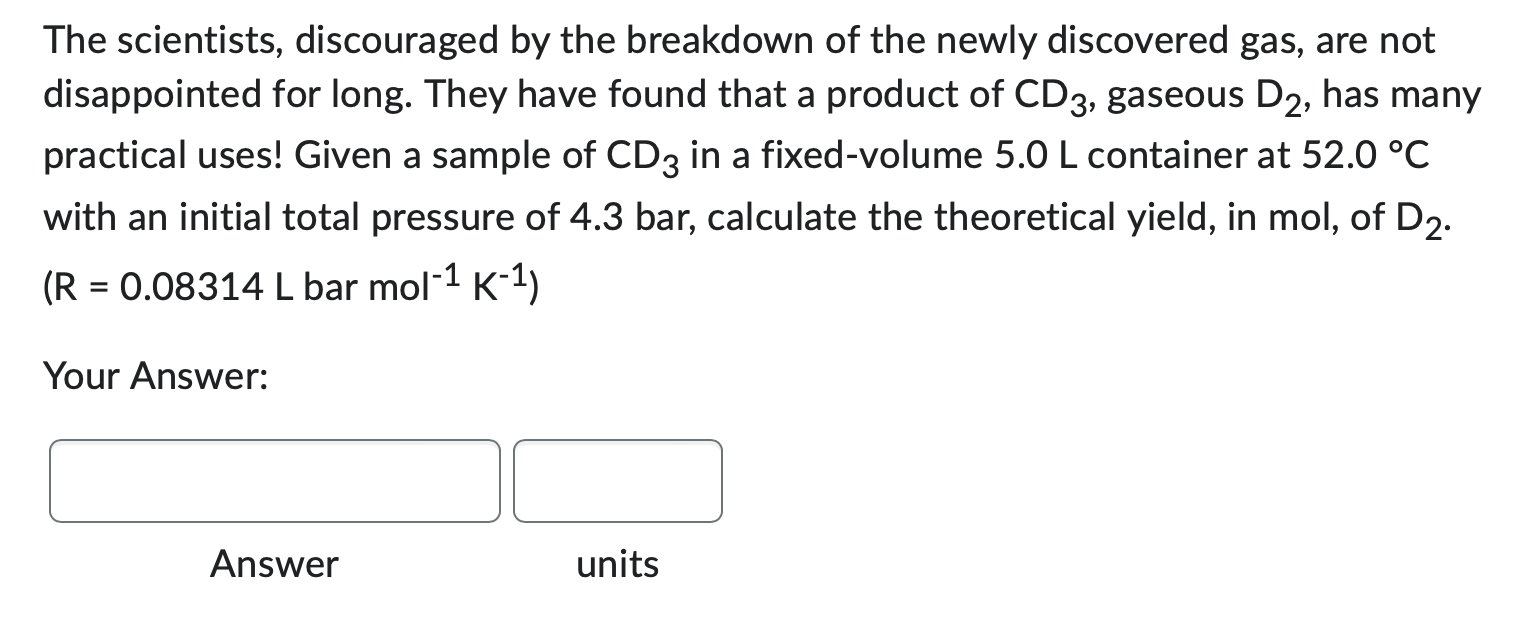
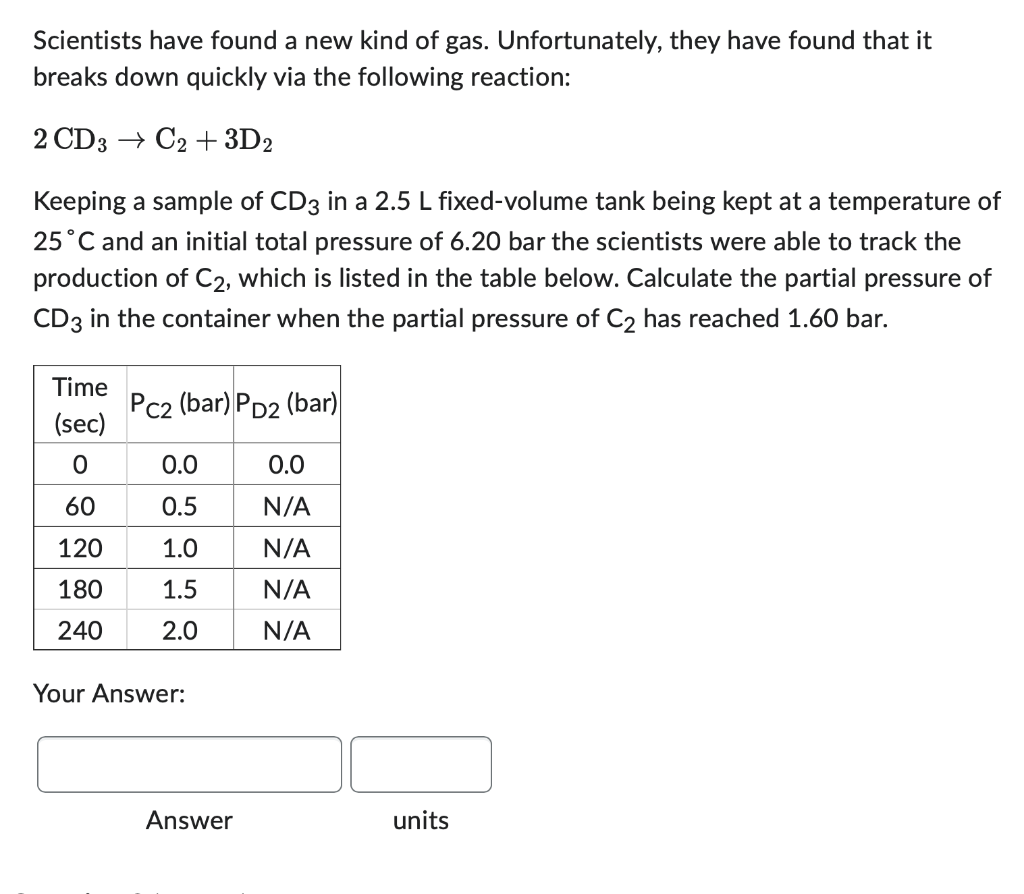
The scientists, discouraged by the breakdown of the newly discovered gas, are not disappointed for long. They have found that a product of CD3, gaseous D2, has many practical uses! Given a sample of CD3 in a fixed-volume 5.0L container at 52.0C with an initial total pressure of 4.3 bar, calculate the theoretical yield, in mol, of D2. (R=0.08314Lbarmol1K1) Your Answer: Answer units Scientists have found a new kind of gas. Unfortunately, they have found that it breaks down quickly via the following reaction: 2CD3C2+3D2 Keeping a sample of CD3 in a 2.5L fixed-volume tank being kept at a temperature of 25C and an initial total pressure of 6.20 bar the scientists were able to track the production of C2, which is listed in the table below. Calculate the partial pressure of CD3 in the container when the partial pressure of C2 has reached 1.60 bar. Your
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


