Question: need help please GENERAL CHEMISTRY 2 SECOND QUARTER Tell the type of process (spontaneous, non-spontaneous, or equilibrium) for each of the value of Gibbs free
need help please
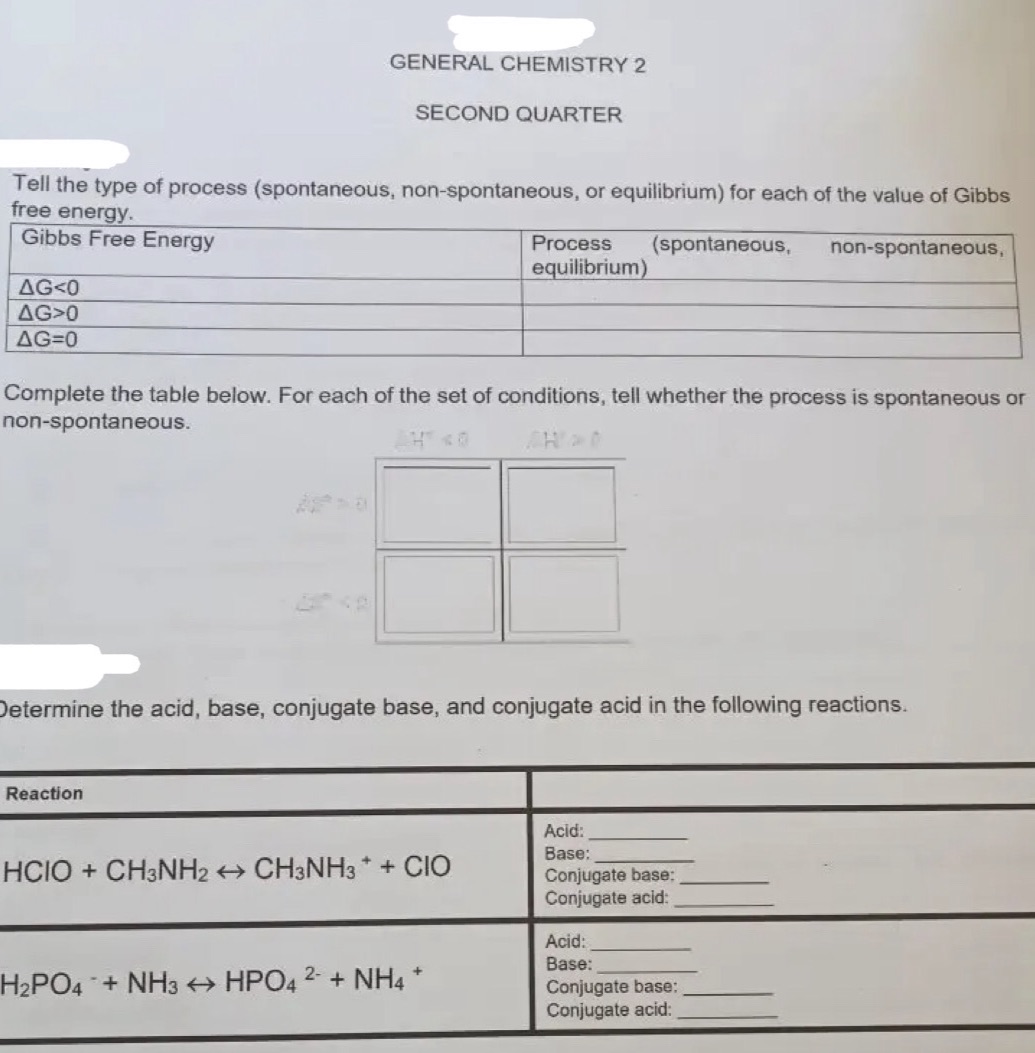
GENERAL CHEMISTRY 2 SECOND QUARTER Tell the type of process (spontaneous, non-spontaneous, or equilibrium) for each of the value of Gibbs free energy. Gibbs Free Energy Process (spontaneous, non-spontaneous, equilibrium) AG0 AG=0 Complete the table below. For each of the set of conditions, tell whether the process is spontaneous or non-spontaneous. Determine the acid, base, conjugate base, and conjugate acid in the following reactions. Reaction Acid: Base: HCIO + CH3NH2 CH3NH3 + + CIO Conjugate base: Conjugate acid: Acid: Base: H2PO4 + NH3 HPO4 2- + NH4 + Conjugate base: Conjugate acid
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


