Question: Need help! Question 2 Using the Bohr model of the atom and Slater's rules, calculate the second ionization energy of sodium (Na). b) Explain why
Need help!
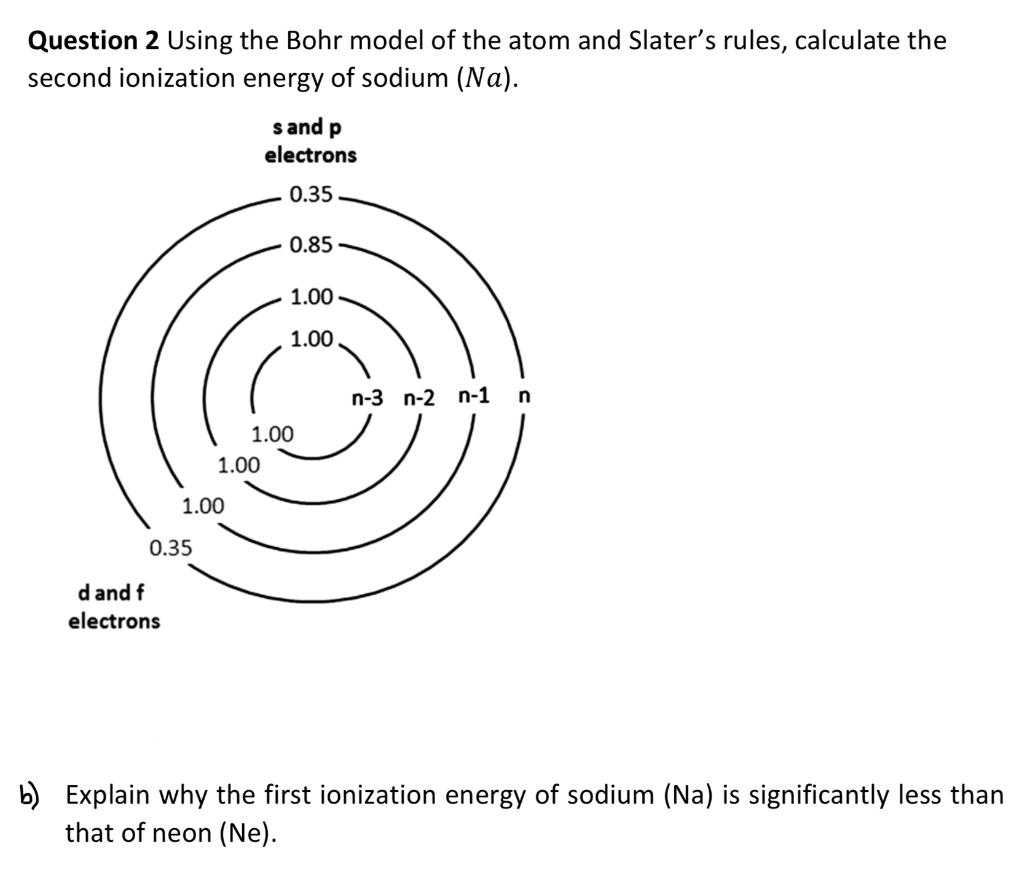
Question 2 Using the Bohr model of the atom and Slater's rules, calculate the second ionization energy of sodium (Na). b) Explain why the first ionization energy of sodium (Na) is significantly less than that of neon (Ne)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


