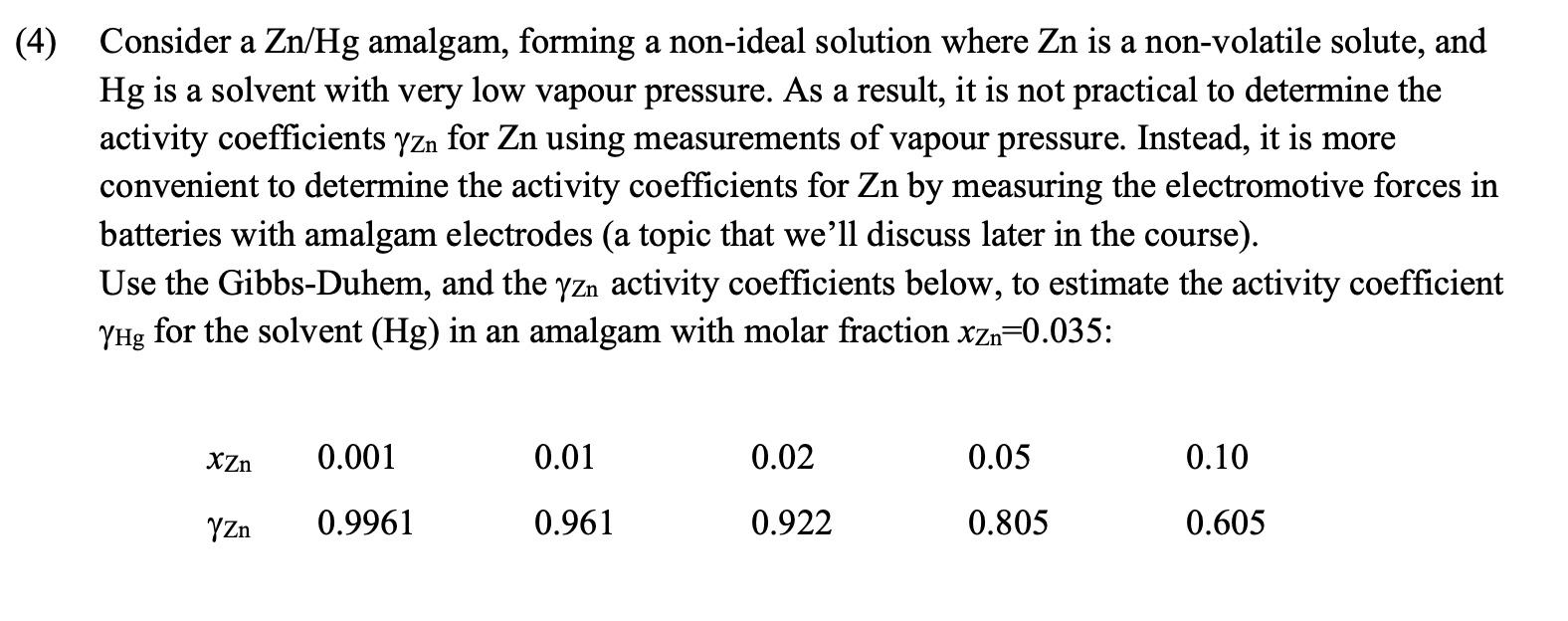
Question: Need help solving this problem in full (please do not copy answer from another chegg question, they are wrong) Consider a Zn/Hg amalgam, forming a
 Need help solving this problem in full (please do not copy answer from another chegg question, they are wrong)
Need help solving this problem in full (please do not copy answer from another chegg question, they are wrong)
Consider a Zn/Hg amalgam, forming a non-ideal solution where Zn is a non-volatile solute, and Hg is a solvent with very low vapour pressure. As a result, it is not practical to determine the activity coefficients Zn for Zn using measurements of vapour pressure. Instead, it is more convenient to determine the activity coefficients for Zn by measuring the electromotive forces in batteries with amalgam electrodes (a topic that we'll discuss later in the course). Use the Gibbs-Duhem, and the Zn activity coefficients below, to estimate the activity coefficient Hg for the solvent (Hg) in an amalgam with molar fraction xZn=0.035
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


